Please contribute research with proper references and links, and/or experiences related to the hormones involved in erectile dysfunction and low libido.
Notes on Testosterone and ED/Libido:
Although the role of androgens in erectile function in men is controversial, primary or secondary hypogonadism is considered key in the pathophysiology of erectile dysfunction (ED) (Korenman et al, 1990; Aversa et al, 2004; Yassin and Saad, 2007). Androgens exert not only genomic effects, for example, by stimulating the expression of the neuronal isoform of nitric oxide (NO) synthase (Reilly et al, 1997; Park et al, 1999), but also nongenomic effects, for example, by relaxing the smooth musculature of coronary arteries and the aorta (Yue et al, 1995; Deenadayalu et al, 2001).
J Androl 2008;29:514–523
T is important for modulating the central and peripheral regulation of ED. Erectile function depends on a normal penile anatomy and a functioning venoocclusive mechanism, which implies integrity of the
structural and cellular components. It was demonstrated that T deprivation causes apoptosis of cells from cavernosal and spongiosal tissues, which can be prevented by androgen administration (Podlasek et al, 2005).
J Androl 2008;29:514–523
Smooth muscle, a vital component of the penile anatomy, is a critical structure for tumescence. Using a rat model, investigators established that experimental castration caused significant reduction in trabecular smooth muscle and a significant increase in connective tissue deposition concomitant with loss of erectile function, indicating that T is vital for smooth muscle integrity (Traish et al, 2003). Further support for the androgenic requirement for penile erection derives from scientific knowledge of the molecular basis for NO function in the penis. It was shown that the NO pathway plays a critical role in initiation and maintenance of erectile function (Burnett, 2004). In animals, the expression of NO synthase (NOS) isoforms in the Canguven and Burnett N 5 a-reductase Inhibitors on Erection 515 corpus cavernosum is regulated by androgens. Researchers found that NOS activity is decreased in
erectile tissues of castrated animals, as is the erectile response to pelvic nerve stimulation (Lugg et al, 1995; Park et al, 1999; Baba et al, 2000). These investigators further established that T restores the erectile response and normalizes NOS protein expression and activity. On the whole, the mechanism of penile erection is a function of corporal smooth muscle relaxation required for blood filling and engorgement of the penis in response to sexual excitement, exerted at the molecular level by cGMP by way of its effector cGKI (Andersson, 2001). Type 5 phosphodiesterase enzyme (PDE5), the predominant phosphodiesterase expressed in the corpus cavernosum, has regulatory control of penile vasorelaxant actions. In the animal model, castration resulted in reduced protein expression and activity of PDE5, although androgen treatment up-regulated the expression of PDE5 activity (Morelli et al, 2004). In addition, the efficacy of PDE5 inhibitors to elicit erections induced by electrostimulation of the cavernous nerves following castration was decreased. After castrated animals were treated with T, the tissue relaxation caused by PDE5 inhibitors was successfully restored (Traish et al, 1999; Morelli et al, 2004). Clinical studies also indicate the direct influence of androgens on erectile responses to PDE5 inhibitors. Investigators carrying out clinical trials in androgen deficient men confirmed these observations and found that with androgen substitution these men displayed enhanced responses to the ED treatment (Aversa et al, 2004; Shabsigh et al, 2004; Morelli et al, 2007). These studies strongly suggest that T exerts vital physiological actions in erectile function.
J Androl 2008;29:514–523
The data suggested that DHT is the active androgen that prevents erectile failure in castrated rats.
With recent discoveries of PDE5 inhibitors as well NOS signaling in the penis, Park et al (1999) examined whether DHT influences the erectile response and the mRNA expressions of NOS isoforms in the penile corpus cavernosum of castrated rats. For this purpose, rats were separated into 5 groups: sham, castrated alone, and castrated receiving T, DHT, or T with the 5ARI finasteride. Both T and DHT effectively restored the erectile response to normal. NOS activity and the amount of neuronal NOS (nNOS) mRNA were also reduced in castrated rats but restored by both T and DHT replacement.
J Androl 2008;29:514–523
On the other hand, T is more relevant than DHT in erectile function, which requires central and peripheral androgenic activity. T exerts both humoral endocrine and local paracrine effects. It is likely that androgens are vital for the development, maintenance and function of penile tissue and regulation of erectile physiology. However, the critical androgenic substance for these effects is most likely T rather than DHT.
J Androl 2008;29:514–523
______________________________________
Notes on Prolactin and ED/Libido:
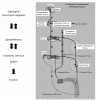
Hyperprolactinemia induces hypogonadism by interfering with the secretion of gonadotropinreleasing
hormone (GnRH) from the hypothalamus (Figure 2).4,5 The resulting decrease in serum T is believed
to be the cause of the erectile dysfunction, although there may be an end-organ effect of prolactin on
the penis.4 Surprisingly, not everyone with hyperprolactinemia has a low serum T level or complains of erectile dysfunction.6,7 However, when the serum prolactin is corrected in patients with an elevated prolactin level and a low serum T level, the serum T level usually returns to a normal value, and erectile function is usually restored (if erectile dysfunction was present).2 Simply treating the patient with exogenous T does not usually correct the erectile dysfunction (unless the prolactin levels are returned to normal). Hyperprolactinemia is a very rare cause of impotence in a general population
of men with impotence.8 However, men who have hyperprolactinemia have a high incidence of
sexual dysfunction, and the erectile dysfunction appears more likely to resolve in patients with the most
severe hyperprolactinemia once this glandular disorder is corrected.7
Main Points
• The prolactin level should be measured in men presenting with a complaint of
erectile dysfunction who have a low serum testosterone level.
• Hypogonadism is almost always the cause of an endocrinopathy that affects
erectile function.
• In hyperprolactinemia, which induces hypogonadism, the excess prolactin
interferes with secretion of gonadotropin-releasing hormone, resulting in
decreased testosterone and erectile dysfunction.
• Hyperprolactinemia caused by a pituitary tumor can be managed with surgery
and/or a dopamine agonist.
Hyperprolactinemia and Erectile Dysfunction
Scott I. Zeitlin, MD, Jacob Rajfer, MD
University of California, Los Angeles, School of Medicine
http://www.ncbi.nlm.nih.gov/pmc/arti...02001_0039.pdf
___________________________________________
Notes on High Estradiol and ED/Libido:
Results of this investigation indicate that oestradiol causes pathophysiological changes in erectile function. These observations provide an indirect evidence for the possible sexual health hazards in man upon inadvertent exposure to environmental oestrogens, ageing and derangement of E2–T ratio.
International Journal of Impotence Research (2003) 15, 38–43. doi:10.1038/sj.ijir.3900945
________________________________________________________
Bonus Feature: Trenbolone and It's Systemic Effects
1. Adrenal Gland
Trenbolone has been implicated in shrinking the adrenal gland in vivo, and antiglucocorticoid activity via inhinbition of dexamethasone-induced transcriptional activity.
The adrenal cortex is regulated by neuroendocrine hormones secreted by the pituitary gland and hypothalamus ? Adrenal insufficiency can also occur when the hypothalamus or the pituitary gland, both located at the base of the skull, does not make adequate amounts of the hormones that assist in regulating adrenal function. This is called secondary adrenal insufficiency and is caused by lack of production of ACTH in the pituitary or lack of CRH in the hypothalamus.
2. Androgen Receptors, Progesterone Receptors
Trenbolone shows strong binding to the androgen receptor, to the progestin receptor and to the glucocorticoid receptor (21). Concerning the androgenic activity, it can be assumed that trenbolone acts like other androgens - in comparison with the most active endogenous hormone dihydrotestosterone (RBA= 100) the affinity of trenbolone-17P is even higher (RBA= 109).
“Biochemistry and physiology of anabolic hormones used for improvement of meat production” Review article, Heinrich Meyer
3. Pituitary, Hypothalamus, Testis
Since trenbolone possesses both estrogenic (ER) or progestogenic (PR) activity, it inhibits LH & FSH by directly down-regulating the GnRH receptors on the pituitary, while also reducing GnRH release from the hypothalamus. Therefore, progestin based AAS such as trenbolone (and nandralone) are “double suppressive” because they are binding to the AR and PR and suppressing LH & FSH by two different mechanisms.
Patterns of LH secretion in castrated bulls during intravenous infusion of androgenic and estrogenic steroids: Pituitary response to exogenous luteinizing hormone-releasing hormone
M.J. D’occhio et al. Biology of reproduction 26, 249-257 (1982)
Studies on the role of sex steroids in the feedback control of FSH concentrations in men.
Sherins RJ, Loriaux DL. 1973 J Clin Endocrinol Metab. 36:886-893
So, What Does This Mean?
1. In terms of Erectile Dysfunction
"On the other hand, T is more relevant than DHT in erectile function, which requires central and peripheral androgenic activity. T exerts both humoral endocrine and local paracrine effects. It is likely that androgens are vital for the development, maintenance and function of penile tissue and regulation of erectile physiology. However, the critical androgenic substance for these effects is most likely T rather than DHT." J Androl 2008;29:514–523
This implies that trenbolone affects and/or displaces testosterone and DHT from androgen receptors, thereby effecting erections.
Further, the increases in prolactin seen on -- and after cessation of -- trenbolone have an adverse, inhibitory effect on testosterone and LH levels. High prolactin is correlated with low libido and erectile dysfunction.
Notes on Testosterone and ED/Libido:
Although the role of androgens in erectile function in men is controversial, primary or secondary hypogonadism is considered key in the pathophysiology of erectile dysfunction (ED) (Korenman et al, 1990; Aversa et al, 2004; Yassin and Saad, 2007). Androgens exert not only genomic effects, for example, by stimulating the expression of the neuronal isoform of nitric oxide (NO) synthase (Reilly et al, 1997; Park et al, 1999), but also nongenomic effects, for example, by relaxing the smooth musculature of coronary arteries and the aorta (Yue et al, 1995; Deenadayalu et al, 2001).
J Androl 2008;29:514–523
T is important for modulating the central and peripheral regulation of ED. Erectile function depends on a normal penile anatomy and a functioning venoocclusive mechanism, which implies integrity of the
structural and cellular components. It was demonstrated that T deprivation causes apoptosis of cells from cavernosal and spongiosal tissues, which can be prevented by androgen administration (Podlasek et al, 2005).
J Androl 2008;29:514–523
Smooth muscle, a vital component of the penile anatomy, is a critical structure for tumescence. Using a rat model, investigators established that experimental castration caused significant reduction in trabecular smooth muscle and a significant increase in connective tissue deposition concomitant with loss of erectile function, indicating that T is vital for smooth muscle integrity (Traish et al, 2003). Further support for the androgenic requirement for penile erection derives from scientific knowledge of the molecular basis for NO function in the penis. It was shown that the NO pathway plays a critical role in initiation and maintenance of erectile function (Burnett, 2004). In animals, the expression of NO synthase (NOS) isoforms in the Canguven and Burnett N 5 a-reductase Inhibitors on Erection 515 corpus cavernosum is regulated by androgens. Researchers found that NOS activity is decreased in
erectile tissues of castrated animals, as is the erectile response to pelvic nerve stimulation (Lugg et al, 1995; Park et al, 1999; Baba et al, 2000). These investigators further established that T restores the erectile response and normalizes NOS protein expression and activity. On the whole, the mechanism of penile erection is a function of corporal smooth muscle relaxation required for blood filling and engorgement of the penis in response to sexual excitement, exerted at the molecular level by cGMP by way of its effector cGKI (Andersson, 2001). Type 5 phosphodiesterase enzyme (PDE5), the predominant phosphodiesterase expressed in the corpus cavernosum, has regulatory control of penile vasorelaxant actions. In the animal model, castration resulted in reduced protein expression and activity of PDE5, although androgen treatment up-regulated the expression of PDE5 activity (Morelli et al, 2004). In addition, the efficacy of PDE5 inhibitors to elicit erections induced by electrostimulation of the cavernous nerves following castration was decreased. After castrated animals were treated with T, the tissue relaxation caused by PDE5 inhibitors was successfully restored (Traish et al, 1999; Morelli et al, 2004). Clinical studies also indicate the direct influence of androgens on erectile responses to PDE5 inhibitors. Investigators carrying out clinical trials in androgen deficient men confirmed these observations and found that with androgen substitution these men displayed enhanced responses to the ED treatment (Aversa et al, 2004; Shabsigh et al, 2004; Morelli et al, 2007). These studies strongly suggest that T exerts vital physiological actions in erectile function.
J Androl 2008;29:514–523
The data suggested that DHT is the active androgen that prevents erectile failure in castrated rats.
With recent discoveries of PDE5 inhibitors as well NOS signaling in the penis, Park et al (1999) examined whether DHT influences the erectile response and the mRNA expressions of NOS isoforms in the penile corpus cavernosum of castrated rats. For this purpose, rats were separated into 5 groups: sham, castrated alone, and castrated receiving T, DHT, or T with the 5ARI finasteride. Both T and DHT effectively restored the erectile response to normal. NOS activity and the amount of neuronal NOS (nNOS) mRNA were also reduced in castrated rats but restored by both T and DHT replacement.
J Androl 2008;29:514–523
On the other hand, T is more relevant than DHT in erectile function, which requires central and peripheral androgenic activity. T exerts both humoral endocrine and local paracrine effects. It is likely that androgens are vital for the development, maintenance and function of penile tissue and regulation of erectile physiology. However, the critical androgenic substance for these effects is most likely T rather than DHT.
J Androl 2008;29:514–523
______________________________________
Notes on Prolactin and ED/Libido:
Hyperprolactinemia induces hypogonadism by interfering with the secretion of gonadotropinreleasing
hormone (GnRH) from the hypothalamus (Figure 2).4,5 The resulting decrease in serum T is believed
to be the cause of the erectile dysfunction, although there may be an end-organ effect of prolactin on
the penis.4 Surprisingly, not everyone with hyperprolactinemia has a low serum T level or complains of erectile dysfunction.6,7 However, when the serum prolactin is corrected in patients with an elevated prolactin level and a low serum T level, the serum T level usually returns to a normal value, and erectile function is usually restored (if erectile dysfunction was present).2 Simply treating the patient with exogenous T does not usually correct the erectile dysfunction (unless the prolactin levels are returned to normal). Hyperprolactinemia is a very rare cause of impotence in a general population
of men with impotence.8 However, men who have hyperprolactinemia have a high incidence of
sexual dysfunction, and the erectile dysfunction appears more likely to resolve in patients with the most
severe hyperprolactinemia once this glandular disorder is corrected.7
Main Points
• The prolactin level should be measured in men presenting with a complaint of
erectile dysfunction who have a low serum testosterone level.
• Hypogonadism is almost always the cause of an endocrinopathy that affects
erectile function.
• In hyperprolactinemia, which induces hypogonadism, the excess prolactin
interferes with secretion of gonadotropin-releasing hormone, resulting in
decreased testosterone and erectile dysfunction.
• Hyperprolactinemia caused by a pituitary tumor can be managed with surgery
and/or a dopamine agonist.
Hyperprolactinemia and Erectile Dysfunction
Scott I. Zeitlin, MD, Jacob Rajfer, MD
University of California, Los Angeles, School of Medicine
http://www.ncbi.nlm.nih.gov/pmc/arti...02001_0039.pdf
___________________________________________
Notes on High Estradiol and ED/Libido:
Results of this investigation indicate that oestradiol causes pathophysiological changes in erectile function. These observations provide an indirect evidence for the possible sexual health hazards in man upon inadvertent exposure to environmental oestrogens, ageing and derangement of E2–T ratio.
International Journal of Impotence Research (2003) 15, 38–43. doi:10.1038/sj.ijir.3900945
________________________________________________________
Bonus Feature: Trenbolone and It's Systemic Effects
1. Adrenal Gland
Trenbolone has been implicated in shrinking the adrenal gland in vivo, and antiglucocorticoid activity via inhinbition of dexamethasone-induced transcriptional activity.
The adrenal cortex is regulated by neuroendocrine hormones secreted by the pituitary gland and hypothalamus ? Adrenal insufficiency can also occur when the hypothalamus or the pituitary gland, both located at the base of the skull, does not make adequate amounts of the hormones that assist in regulating adrenal function. This is called secondary adrenal insufficiency and is caused by lack of production of ACTH in the pituitary or lack of CRH in the hypothalamus.
2. Androgen Receptors, Progesterone Receptors
Trenbolone shows strong binding to the androgen receptor, to the progestin receptor and to the glucocorticoid receptor (21). Concerning the androgenic activity, it can be assumed that trenbolone acts like other androgens - in comparison with the most active endogenous hormone dihydrotestosterone (RBA= 100) the affinity of trenbolone-17P is even higher (RBA= 109).
“Biochemistry and physiology of anabolic hormones used for improvement of meat production” Review article, Heinrich Meyer
3. Pituitary, Hypothalamus, Testis
Since trenbolone possesses both estrogenic (ER) or progestogenic (PR) activity, it inhibits LH & FSH by directly down-regulating the GnRH receptors on the pituitary, while also reducing GnRH release from the hypothalamus. Therefore, progestin based AAS such as trenbolone (and nandralone) are “double suppressive” because they are binding to the AR and PR and suppressing LH & FSH by two different mechanisms.
Patterns of LH secretion in castrated bulls during intravenous infusion of androgenic and estrogenic steroids: Pituitary response to exogenous luteinizing hormone-releasing hormone
M.J. D’occhio et al. Biology of reproduction 26, 249-257 (1982)
Studies on the role of sex steroids in the feedback control of FSH concentrations in men.
Sherins RJ, Loriaux DL. 1973 J Clin Endocrinol Metab. 36:886-893
So, What Does This Mean?
1. In terms of Erectile Dysfunction
"On the other hand, T is more relevant than DHT in erectile function, which requires central and peripheral androgenic activity. T exerts both humoral endocrine and local paracrine effects. It is likely that androgens are vital for the development, maintenance and function of penile tissue and regulation of erectile physiology. However, the critical androgenic substance for these effects is most likely T rather than DHT." J Androl 2008;29:514–523
This implies that trenbolone affects and/or displaces testosterone and DHT from androgen receptors, thereby effecting erections.
Further, the increases in prolactin seen on -- and after cessation of -- trenbolone have an adverse, inhibitory effect on testosterone and LH levels. High prolactin is correlated with low libido and erectile dysfunction.
Last edited: