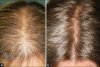
Finasteride in the Treatment of Female Pattern (Androgenic) Alopecia

Oliveira-Soares R, JM ES, Correia MP, Andre MC. Finasteride 5 mg/day Treatment of Patterned Hair Loss in Normo-androgenetic Postmenopausal Women. Int J Trichology 2013;5(1):22-5. Finasteride 5 mg/day treatment of patterned hair loss in normo-androgenetic postmenopausal women Oliveira-Soares R, e Silva J M, Correia M P, André MC - Int J Trichol
BACKGROUND: There is no consensus on the standard treatment options for female pattern androgenetic alopecia (AGA). Efficacy of finasteride in women is controversial. The purpose of this study was to evaluate the clinical efficacy and safety of 5 mg/day oral finasteride in normoandrogenic postmenopausal woman.
MATERIALS AND METHODS: A total of 40 normoandrogenic postmenopausal women with AGA was enrolled in this study. They were treated with oral finasteride 5 mg/day for 18 months. Efficacy was evaluated by patient's satisfaction and global photograph assessment. All the 40 patients completed 18 months of finasteride treatment schedule.
RESULTS: After 6 months, 22 patients referred significant improvement, 12 moderate improvement, and 6 no improvement. Regarding to global photo assessment, 8 patients showed no improvement, 16 showed moderate improvement and 16 showed significant improvements at the 6(th) month. A slight improvement was observed over time from 6 to 12 and 18 months observation. Maintained libido reduction was referred by four patients and liver enzymes increase was observed in one patient. Older patients were more prone to worse response.
DISCUSSION: Finasteride 5 mg/day is effective and safe for the treatment of female AGA in postmenopausal women in the absence of clinical or laboratory signs of hyper-androgenism.
Oliveira-Soares R, JM ES, Correia MP, Andre MC. Finasteride 5 mg/day Treatment of Patterned Hair Loss in Normo-androgenetic Postmenopausal Women. Int J Trichology 2013;5(1):22-5. Finasteride 5 mg/day treatment of patterned hair loss in normo-androgenetic postmenopausal women Oliveira-Soares R, e Silva J M, Correia M P, André MC - Int J Trichol
BACKGROUND: There is no consensus on the standard treatment options for female pattern androgenetic alopecia (AGA). Efficacy of finasteride in women is controversial. The purpose of this study was to evaluate the clinical efficacy and safety of 5 mg/day oral finasteride in normoandrogenic postmenopausal woman.
MATERIALS AND METHODS: A total of 40 normoandrogenic postmenopausal women with AGA was enrolled in this study. They were treated with oral finasteride 5 mg/day for 18 months. Efficacy was evaluated by patient's satisfaction and global photograph assessment. All the 40 patients completed 18 months of finasteride treatment schedule.
RESULTS: After 6 months, 22 patients referred significant improvement, 12 moderate improvement, and 6 no improvement. Regarding to global photo assessment, 8 patients showed no improvement, 16 showed moderate improvement and 16 showed significant improvements at the 6(th) month. A slight improvement was observed over time from 6 to 12 and 18 months observation. Maintained libido reduction was referred by four patients and liver enzymes increase was observed in one patient. Older patients were more prone to worse response.
DISCUSSION: Finasteride 5 mg/day is effective and safe for the treatment of female AGA in postmenopausal women in the absence of clinical or laboratory signs of hyper-androgenism.