Hey mate, any chance you can give me a test e recipe for say 50ml of test e? I’m completely newI'm sorry did I read that melting point rarely comes into concern when brewing?!?!?!? You absolutely are supposed to heat to melting point then hold it there while stiring during the brew process. In fairness not every hormone will crash without being heated to .melting point but enough will. Have you ever had high concentration test e (over 300mgs) that leaves knot in your ass.....that's because the brewer didn't heat to melting and hold it there while stiring for long enough. UGLs rely too much on solvents and EO. They could get actual high concentration products with no more than 20% bb if they simply heated it high enough and long enough while stiring it.
MELTING POINTS are ABSOLUTELY important to educated brewers!!!
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
Style variation
Guest viewing limit reached
- You have reached the maximum number of guest views allowed
- Please register below to remove this limitation
- Already a member? Click here to login
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Melting point
- Thread starter Hulk4
- Start date
theotherguy
Member
There are recipes all over the homebrew section, just search test e recipes and I bet you'll find one pretty quick. You can do that for most raws.Hey mate, any chance you can give me a test e recipe for say 50ml of test e? I’m completely new
Here's a recipe for test E 250. That BA/BB ratio can be changed a bit too, but 2/18 is pretty common. (first recipe is on me, you gotta search for any others)
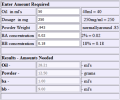
Theotherguy already covered what I would have said about the ratios (the real part of a recipe, the rest is math for volumes). There is no real need to use any exotic carriers unless you want to try something like 400mg per ml. For anything up to the 300 range you can use whatever. I personally like MCT oil and mig because they are thin plus mig can almost act like a solvent in a way sometimes. Now at any concentration I would recommend you just put your carrier oil and raw powder together first. Then heat and stir that until you are up around the boiling temperature of water, dont listen to the people who say this damages the hormone, it does not in anyway. I would keep it at that temp and stirring for at least half an hour. Now you should have top quality raws but unfortunately with how the Chinese have to manufacture these hormones at these prices sometimes the final wash does not get all the leftover carbolic acids. That temperature of around the boiling temperature of water (I actually prefer up around 225f vs 212f. ) is the temp where the carbolic acids boil off. Now there are a range of temps for the different carbolic acids but this temp range is an old Homebrewer pass down. If you do have the leftover carbolics in your raws then you brew them they can most definitely cause PIP. I can tell you from experience that the batchs I did without heating and holding it for half an hour definitely left a knot in my ass. The batches I have done with the heat and hold are painless.Hey mate, any chance you can give me a test e recipe for say 50ml of test e? I’m completely new
The other part of the heating and stirring is it fully melts the hormone out of the crystalline structure. Yeah the benzyl benzoate is supposed to help dissolve the hormone but really it is just there to help prevent it from reverting to the crystalline structure.
If you do decide to try 400mgs per ml then I would suggest using mig or MCT oil and if possible use some ethyl Oleate. Also for 400 I would up the ratio of benzyl benzoate to 20%.
BawlsDeep
Subscriber
Categorically incorrect. Excepting some long ester hormones, heating to the melting point will cause oxidation,polymerization, or outright cook and destroy the compound. Same applies if you heat it to EO bp.And NO you will not hurt the hormone if you go past .melting point. You can go well past it. You NEED to heat it past melting point. As far as how far past....your eo will evaporate well before you get to the danger zone.
No, sterilization comes from filtering. BA maintains the sterility. Again,wrong.Does 65c sterilize the solution????????? NO!!!
Please try heating to a higher temp, does not have to be a crazy temp just high enough to sterilize the solution. Seriously the heating part of the brew is important to guarantee a sterile finished product.
Keeps it sterile, a preservativebenzyl alcohol sterilizes the solution.
CorrectBenzyl alcohol keeps things from growing. It CAN kill things but that is not the reason it is added, it's a preservative that keeps things from growing.
Good advice, use it.
I urge you to continue your quest for knowledge.
Heating too much will do thisdon't destroy the oil or raws when brewing
Ohh sweet ironyMan I feel bad for just totally schooling you like that all based off an assumption. I realized afterwards that I didn't find out if English is a second language or if you have a learning/cognitive disorder.
Oxidation and polymerization for starters. Melting and solvation are not the same, learn the difference.And if some raws don't even melt until over 100 Celsius then how would heating them to just sterilizing temps hurt them?? I can tell you from volume experience that there is no estered hormone you can damage by hearing it to sterilizing temps again quite a few don't even melt until well past 100celsius.
You are completely wrong and full of shit. You never heated a hormone base or short ester to its melting point.
Idiot or liar? Probably both.
Fine for test enanthate maybe, if u say so. I never usedan enanthanate. But Try this with any trenbolone compound. Probably boldenone too. Ull watch it darken before your eyes.I always heat it to about 225-230f, and auto stir for about 45minutes to an hour. I have never had any sort of PIP again and I went back to just 2%ba, 18%bb and rest mct or mig.
Heat until it disolves,no higher. Stop heating and reevaluate ur solvents and oil choice if u have to heat until it darkens to get it into solution.Then heat and stir that until you are up around the boiling temperature of water, dont listen to the people who say this damages the hormone, it does not in anyway.
Heat absolutely does damage hormones.
HilariousYeah the benzyl benzoate is supposed to help dissolve the hormone but really it is just there to help prevent it from reverting to the crystalline structure.
I learned from an actual pharmacist producer....where did you learn?Categorically incorrect. Excepting some long ester hormones, heating to the melting point will cause oxidation,polymerization, or outright cook and destroy the compound. Same applies if you heat it to EO bp.
No, sterilization comes from filtering. BA maintains the sterility. Again,wrong.
Keeps it sterile, a preservative
Correct
Good advice, use it
Heating too much will do this
Ohh sweet irony
Oxidation and polymerization for starters. Melting and solvation are not the same, learn the difference.
You are completely wrong and full of shit. You never heated a hormone base or short ester to its melting point.
Idiot or liar? Probably both.
Fine for test enanthate maybe, if u say so. I never usedan enanthanate. But Try this with any trenbolone compound. Probably boldenone too. Ull watch it darken before your eyes.
Heat until it disolves,no higher. Stop heating and reevaluate ur solvents and oil choice if u have to heat until it darkens to get it into solution.
Heat absolutely does damage hormones.
Hilarious
And fucking look up the definition of oxidation....start your education with a fucking dictionaryCategorically incorrect. Excepting some long ester hormones, heating to the melting point will cause oxidation,polymerization, or outright cook and destroy the compound. Same applies if you heat it to EO bp.
No, sterilization comes from filtering. BA maintains the sterility. Again,wrong.
Keeps it sterile, a preservative
Correct
Good advice, use it
Heating too much will do this
Ohh sweet irony
Oxidation and polymerization for starters. Melting and solvation are not the same, learn the difference.
You are completely wrong and full of shit. You never heated a hormone base or short ester to its melting point.
Idiot or liar? Probably both.
Fine for test enanthate maybe, if u say so. I never usedan enanthanate. But Try this with any trenbolone compound. Probably boldenone too. Ull watch it darken before your eyes.
Heat until it disolves,no higher. Stop heating and reevaluate ur solvents and oil choice if u have to heat until it darkens to get it into solution.
Heat absolutely does damage hormones.
Hilarious
Categorically incorrect. Excepting some long ester hormones, heating to the melting point will cause oxidation,polymerization, or outright cook and destroy the compound. Same applies if you heat it to EO bp.
I don't know what your formation is but... Heating gear to it's boiling point is correct.
Everything has a boiling point, you need to heat what needs to be heat and at the right temp.
BA BB MCT oil, have all boiling points much higher than AAS, so yes you can heat AAS safely to boiling point and obtain a perfect homebrewed gear.
BawlsDeep
Subscriber
I think you are too fucking stupid and arrogant to help, but i hope nobody reads your moronic ramblings and mistakenly thinks you have the faintest clue what ure talking about.And fucking look up the definition of oxidation....start your education with a fucking dictionary
What does your dictionary give for 'carbolic acid', since u throw it around in this thread. Moron.
Kindly tell me the boiling points of some steroids, and also the bp of BA, BB, and MCT.I don't know what your formation is but... Heating gear to it's boiling point is correct.
Everything has a boiling point, you need to heat what needs to be heat and at the right temp.
BA BB MCT oil, have all boiling points much higher than AAS, so yes you can heat AAS safely to boiling point and obtain a perfect homebrewed gear.
Everything you said was horribly incorrect and I pity anyone who tries to boil their solvents or steroids.
Fuck you if ure trolling and hoping someone would do something so stupid. If you arent trolling please read up on some homebrew techs and the reasons for each step.
If you dont believe me, brew some steroids and heat to boiling. Post before and after pics of the liquid. Ull have a dark polymerized mess at best and possibly a smokey disaster. Dont even think of injecting anything that has turned dark after brewing and/or hasnt been filtered prior to injection.
Here is an article where they heated steroids including Nandrolone to about 300c and noticed substantial decomposition (oxidative degradation they called it) starting about 251c

(PDF) Thermal stability evaluation of doping compounds before GC-MS analysis by DSC
PDF | The Medical Commission of the International Olympic Committee forbids the use of anabolic androgenic steroids, β-agonists, stimulant and narcotic... | Find, read and cite all the research you need on ResearchGate
Qualityoflife86
Member
I have 2 questions.I think you are too fucking stupid and arrogant to help, but i hope nobody reads your moronic ramblings and mistakenly thinks you have the faintest clue what ure talking about.
What does your dictionary give for 'carbolic acid', since u throw it around in this thread. Moron.
Kindly tell me the boiling points of some steroids, and also the bp of BA, BB, and MCT.
Everything you said was horribly incorrect and I pity anyone who tries to boil their solvents or steroids.
Fuck you if ure trolling and hoping someone would do something so stupid. If you arent trolling please read up on some homebrew techs and the reasons for each step.
If you dont believe me, brew some steroids and heat to boiling. Post before and after pics of the liquid. Ull have a dark polymerized mess at best and possibly a smokey disaster. Dont even think of injecting anything that has turned dark after brewing and/or hasnt been filtered prior to injection.
Here is an article where they heated steroids including Nandrolone to about 300c and noticed substantial decomposition (oxidative degradation they called it) starting about 251c

(PDF) Thermal stability evaluation of doping compounds before GC-MS analysis by DSC
PDF | The Medical Commission of the International Olympic Committee forbids the use of anabolic androgenic steroids, β-agonists, stimulant and narcotic... | Find, read and cite all the research you need on ResearchGatewww.researchgate.net
I've heard so many ppl say heat up to 80,90,100C for 30 min to an hour and others say you don't need more than 50C. obviously depending on the compound.
so.
how hot do u normally let testE and TestC get to when u start, do you start with the raw powder and solvents first? and heat and stir at temp for 30? 60? minutes?
or
start with the oil and raws first? and heat and stir at temp for 30? 60? minutes
or can you toss it all in at the same time?
Ive heard Tren E should not go over 60C when heating.
BawlsDeep
Subscriber
Ive heard tren will darken if heated, and i would personally toss any darkened brew. Brewing is cheap,dont risk ur health.I have 2 questions.
I've heard so many ppl say heat up to 80,90,100C for 30 min to an hour and others say you don't need more than 50C. obviously depending on the compound.
so.
how hot do u normally let testE and TestC get to when u start, do you start with the raw powder and solvents first? and heat and stir at temp for 30? 60? minutes?
or
start with the oil and raws first? and heat and stir at temp for 30? 60? minutes
or can you toss it all in at the same time?
Ive heard Tren E should not go over 60C when heating.
I have never brewed test E or Cyp, but i too have heard that they can be heated to 80+ with no issue. If you are in a hurry, just put it all together and stir amd heat til 80, it should dissolve quick and be no problem. They will probably dissolve long before hitting 80
I was mainly commenting to keep anyone from trying to heat to the boiling point of these solvents.
A good method that should work for all brews, even tren, is to add everything together and stir with heating to no more than 50c. If it dissolves and doesnt crash u got a good formula amd good brew.
CaliforniaHGH
Subscriber
Use only enough heat to melt it, no more.
Tren can be made without darkening but it requires an expert.
Tren can be made without darkening but it requires an expert.
longstad7282
New Member
Out of the common used ingredients EO is the one that breaks down at the lowest temp, still over 200 celsius. No common oils burn below that and infact most are up or over 300 Celsius. And if some raws don't even melt until over 100 Celsius then how would heating them to just sterilizing
What do u recommend is the best melting point for tren ace and test e so far all I could find is 94-96 for tren and 32-36 for testOk tren ace should end up an amber color (depending on concentration and carrier), tren enanthate will also have a yellow color to its finished product. Even high concentrations of test cypionate can have a yellow tint to the finished product. EQ can have a very very light brown to the tint. Otherwise I can not think of any other hormone/ester combos that should or would automatically have a coloring to the finished product. It does happen, just look at your raw, of it has a color other than white then you can expect it to influence the final products color.
longstad7282
New Member
Where can you find the exact melting points for tren ace and test eMelting point is neccesary. Judt ifbu use another kind of sovlvents , as guaiacol then perhaps u wint need to achieve the melting point but you will need to at least reach below it.
Sterilyty of the oils just when u filter with 0.22 filter.
Ba is a preservative and helps to keep bacteria growing under control.
STERILITY JUST WHEN U FILTER.
Warm oils wont make anything u need like an hour at 150-160 degrees to steril ur oil and mct smoke point is like 150, so u will "Damage" it.
If u filter u r brew will be perfect.
longstad7282
New Member
So this is a test e 250mg/ml recipe correct and what is the recommended melting point I will be using a stove and I have been looking for tren ace 100mg/ml recipes but I can’t dictate Which is the best everybody says one recipe is better than the other how would you go about finding a good legit tren ace recipeThere are recipes all over the homebrew section, just search test e recipes and I bet you'll find one pretty quick. You can do that for most raws.
Here's a recipe for test E 250. That BA/BB ratio can be changed a bit too, but 2/18 is pretty common. (first recipe is on me, you gotta search for any others)
View attachment 165562
longstad7282
New Member
I am using a stove to cook tren ace and test e should I be looking for the boiling point or the melting point ?I don't know what your formation is but... Heating gear to it's boiling point is correct.
Everything has a boiling point, you need to heat what needs to be heat and at the right temp.
BA BB MCT oil, have all boiling points much higher than AAS, so yes you can heat AAS safely to boiling point and obtain a perfect homebrewed gear.
longstad7282
New Member
I did read multiple still could not find my answersJeez man.... Read the threads.
For some compounds/raws, heating to the melting point will destroy the raws. You do not need to heat to the melting point. Just heat until dissolved. The temperature required for that depends on the solubility of the raw in the specific carrier oil you use.
Hearing past the melting point won’t necessarily degrade the raw. Degradation happens near the “decomposition temperature” which is also unique to each compound. The decomposition temperature is generally well above the melting temp of long esters (undecyclonate) because they have very low melting points. but the decomposition temperature can also be well below the melting point of “no-ester”/“base” raws, which have very high melting points. Generally the temperature of decomposition won’t change “much” between different esters of the same compound, but the melting point will.
There’s also not a single “decomposition temperature” per se. Rather, there’s an equation (Arrhenius Equation) which shows that noticeable decomposition happens at all temperatures, just glacially slowly at like 40F, and in a matter of minutes at 250F.
I don’t find comprehensive thermal decomposition data immediately handy but for a general “sense”: https://www.scholarsresearchlibrary...adation-products-by-rapid-rpuplcms-method.pdf
This shows that heating test base to 80C (176F) for 6 hours results in 1.6% of the testosterone degrading. This is probably the highest temperature a high quality UGL would use to try to dissolve it in solution (test base / test no ester is a terrible example because it doesn’t dissolve and is usually injected as a “suspension” in either oil or bac water but whatever just roll with it okay) and all esterified testosterone would need lower temperatures than test base to dissolve. However, heating test base to its melting point, 155C (311F), for 3 hours resulting in 40% of the raw undergoing thermal decomposition. Also some people put their vials in boiling water (100C) when they crash, and that could result in more degradation of testosterone than is desired. I can't find data on how much/how fast.
However, the melting point of testosterone undecanoate is only 60C…so you could heat that to its melting point and expect minimal or no degradation of the testosterone.
Tl;dr: Brewing temperature limit has nothing to do with melting point. There’s a concept of “decomposition temperature” but it’s more of a guideline/curve/rangeand not a strict single number. Just heat to the lowest temperature that the compound dissolved in your oil. Minimize time spent hot too - degradation is caused by “temperature times time”, so minimize both, but if you have to choose between them, temperature is way more important than time.
Also keep your compounds away from UV light for more than a few hours. That consistently degrades raws and oils over time (slowly, but surely).
HGH and other peptides also have a “temperature of denaturization” in addition to “temperature of degradation” but it will be higher than the temperature of degradation and if you’re heating peptides you’re already completely doing it wrong. Effects of thermal and mechanical stress on the physical stability of human growth hormone and epidermal growth factor - PubMed you’ll lose 20% of the hGH by heating it to 70C (50% if you leave it that hot for 3 hours). But it will “denature” at 74C…at which point you will lose 100% of it pretty instantly.
Cooking eggs is an example of denaturing proteins/peptides. It’s not burned, but it’s clearly changed.
Hearing past the melting point won’t necessarily degrade the raw. Degradation happens near the “decomposition temperature” which is also unique to each compound. The decomposition temperature is generally well above the melting temp of long esters (undecyclonate) because they have very low melting points. but the decomposition temperature can also be well below the melting point of “no-ester”/“base” raws, which have very high melting points. Generally the temperature of decomposition won’t change “much” between different esters of the same compound, but the melting point will.
There’s also not a single “decomposition temperature” per se. Rather, there’s an equation (Arrhenius Equation) which shows that noticeable decomposition happens at all temperatures, just glacially slowly at like 40F, and in a matter of minutes at 250F.
I don’t find comprehensive thermal decomposition data immediately handy but for a general “sense”: https://www.scholarsresearchlibrary...adation-products-by-rapid-rpuplcms-method.pdf
This shows that heating test base to 80C (176F) for 6 hours results in 1.6% of the testosterone degrading. This is probably the highest temperature a high quality UGL would use to try to dissolve it in solution (test base / test no ester is a terrible example because it doesn’t dissolve and is usually injected as a “suspension” in either oil or bac water but whatever just roll with it okay) and all esterified testosterone would need lower temperatures than test base to dissolve. However, heating test base to its melting point, 155C (311F), for 3 hours resulting in 40% of the raw undergoing thermal decomposition. Also some people put their vials in boiling water (100C) when they crash, and that could result in more degradation of testosterone than is desired. I can't find data on how much/how fast.
However, the melting point of testosterone undecanoate is only 60C…so you could heat that to its melting point and expect minimal or no degradation of the testosterone.
Tl;dr: Brewing temperature limit has nothing to do with melting point. There’s a concept of “decomposition temperature” but it’s more of a guideline/curve/rangeand not a strict single number. Just heat to the lowest temperature that the compound dissolved in your oil. Minimize time spent hot too - degradation is caused by “temperature times time”, so minimize both, but if you have to choose between them, temperature is way more important than time.
Also keep your compounds away from UV light for more than a few hours. That consistently degrades raws and oils over time (slowly, but surely).
HGH and other peptides also have a “temperature of denaturization” in addition to “temperature of degradation” but it will be higher than the temperature of degradation and if you’re heating peptides you’re already completely doing it wrong. Effects of thermal and mechanical stress on the physical stability of human growth hormone and epidermal growth factor - PubMed you’ll lose 20% of the hGH by heating it to 70C (50% if you leave it that hot for 3 hours). But it will “denature” at 74C…at which point you will lose 100% of it pretty instantly.
Cooking eggs is an example of denaturing proteins/peptides. It’s not burned, but it’s clearly changed.
narta
Member
Destroy is a strong word. Examples with ANY kind of data? Cause I maybe doing this wrong for 2+ decades if that's the case.For some compounds/raws, heating to the melting point will destroy the raws
In my post I noted:Destroy is a strong word. Examples with ANY kind of data? Cause I maybe doing this wrong for 2+ decades if that's the case.
Yeah, you're not gonna heat it for "3 hours". But even if you only lose 10%, what the fuck did it "degrade" or "decompose" into? Are you sure you want to inject that...However, heating test base to its melting point, 155C (311F), for 3 hours resulting in 40% of the raw undergoing thermal decomposition.
Personally I use a hotplate with a temperature probe and a magnetic stirrer. You can set the desired temperature. I set it to 150F to 175F (<80C) and that works great for normal brews. You do need to be careful to make sure the temperature probe is at least 2-3 inches deep though, which is tricky if you're only brewing 100mL or so, but pretty easy for 250mL brews with a 250mL Erlenmeyer flask. It can "overshoot" temperature a bit so I usually set it lower than 175F and then set it higher later.
I have thermocouples to double-check that the temperature reading is correct, because the thermocouples react super instantly to any temperature change.
$57 - Hotplate with Temperature Setting and Magnetic Stirrer (Can be a bit tricky to press the right combination of buttons to get the heating mode to follow temperature rather than just being stuck "ON REALLY HOT HOT HOT HOT HOT" ... best to trial it with a beaker of water first to see if you can get it to stick at 150F)
$10 - Bunch of magnetic stir bars (These are coated in PTFE which is supposed to be compatible with oils, BA, BB, and most other common chemicals)
$30 - Thermocouple reader (Very, very optional)
Last edited:
Similar threads
- Replies
- 1
- Views
- 173

