I decided some time ago I'm not the Headmaster of "Ghoul's Academy of Wayward Knuckledraggers", so I'm reluctant to waste my time with basic education on a given topic, and only take the time to respond with explanations to questions presented in a sincere respectful way.
Since you haven't been a complete asshole, I'll give you the benefit of the doubt,
You have a fundamental misunderstanding of how the threat of immunogenicity is measured, and managed.
The study you quoted does not mean "immunogenicity is not a problem with Tirzapatide no matter how high immunogenicity levels get, Hooray!"
It means "Using the formulation we intend to sell at retail, delivered in these specific pens, at these doses, injected in these 3 areas, ONCE A WEEK, over this period of time, in patients matching the demographics of the selected subjects, the measured level of immunogenicity that developed did not rise to a clinically significant level."
If ANY of those factors change, like the prescribed injection frequency or the plastic used to make the pens, they MUST demonstrate to the FDA that immunogenicity won't increase to a level that threatens health.
It does NOT MEAN "And if we let our manufacturing standards decline inducing higher levels of impurities, synthesized instead of of using recombinant methods to produce Tirz, used higher concentrations, switched to daily injections, left it unrefrigerated for months, let subjects hop on and off at will to maximize "re-exposure events" immunogenicity won't be a problem.
UGL tirz is not recombinant, it's synthesized.
It's not supplied via a cold chain in high quality, particulate free containers.
It's not reconstituted with regard to any specific PH to prevent immunogenic inducing aggregates.
It's not very likely to have a 2 year shelf life in reconstituted form like pharma is, because UGL is "sloppy", allowing for all these factors to degrade it into forms that are known to induce higher levels of immunogenicity.
Here are the in vitro immune responses to pharma GLPs vs 2 compounding pharmacy GLPs, who are using synthesized APIs and excipients that differ from the pharma formulation. Do you think a 200x stronger immune reaction will have the same "non clinically significant" impact the ultra-low immunogenicity pharma products do?
Pharma GLP immune response (left side of each chart vs Compounder GLPs (two on the right of each chart).
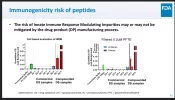
Even in the case of pharma, some GLPs result in significant enough immunogenicity over 6 months or a year diabetics start to see their blood glucose get out of control because they develop immunity to the proteins, and have to stop using the drugs. 200x stronger reactions are certain to bring that on faster and with more severe effects.
All I'm suggesting is doing what you can to minimize the possibility. Take whatever action you're willing to and find reasonable. Notice that after .2um filtration, one of the compounder GLP showed an 80% lower immune response. Would you consider filtering an unnecessary waste of effort?
You're suggesting no need to bother because it can't possibly be a problem. You likely won't be satisfied without a trial intentionally trying to induce immunogenicity in subjects, which would never be allowed, The FDA specifically expresses great concern that trial subjects not be exposed to immunogenic risks, and so the unpublished computer simulations, in vitro tests, and animal studies are performed, then adjustments are made, and only once the FDA is very confident a particular protocol won't be an issue will trials on human subjects be allowed.
We are the *trial subjects* for a whole host of experiments with these unregulated, largely untested novel compounds without the benefit of any immunogenicity predicative testing to ensure it won't impact our health.
Individuals can decide which way is prudent for themselves.






