Type-IIx
Member
Risk-reward profile for progesterone/allopregnanolone supplementation
Author: Type-IIx
It has come to my attention that there is a growing number of bodybuilders and AAS users subscribing to the practice of administering exogenous progesterone or allopregnanolone (or both) as supplemental neurosteroids. I want to put this information out there for them, so that they may balance the tradeoffs of this risky endeavour with appropriate information from different sources.
This article purposely discusses progestins (synthetic) and progesterone receptor agonists, including "bioidentical" progesterone (which is not inherently safe due to its being "bioidentical...") as a singular class of agents that are classic female hormonal agents. A later "article" will be forthcoming that distinguishes between progestins, progesterone, and prolactin (as there is widespread confusion, understandably).
Neurosteroids: steroids that are synthesized in the CNS from cholesterol or sterol precursors [1].
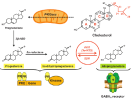
Biosynthesis of allopregnanolone [5].
Allopregnanolone
Allopregnanolone: potent and positive allosteric modulator (PAM) of GABA-A receptors [1], regulates stress, mood, and female sexual behavior [3].
Allopregnanolone exhibits benzodiazepine and antidepressant qualities by modulating GABA-A receptors. Allopregnanolone and progesterone, as steroids, are broad spectrum in their actions, meaning that they affect multiple systems in unpredictable ways.
Progesterone
The "mother molecule"
Progesterone and its metabolites are neurosteroids that also play an important role in myelination. Consequently, blocking the production of the metabolites of progesterone has been found to have an adverse effect on the myelination process. In rodent and in vitro models, there is evidence of therapeutic benefits for certain types of injury (ischemic stroke, ALS, MS, carpal tunnel syndrome) (22) [6]. Progesterone is upregulated in TBI and stroke patients.
Thus, exogenous progesterone likely has some efficacy in recovery from TBI and stroke, and perhaps for demyelinating disorders. It also has demonstrable efficacy in the treatment of postpartum depression.
Micronized progesterone
Micronised P4 (but not synthetic progestins) and one of its major metabolites, allopregnanolone, have been shown to modulate GABAergic transmission with a similar potency or even greater efficacy, than those of alcohol, benzodiazepines, or barbiturates (4) [6].
P4 is a weak agonist for the GR and AR, has no significant activity via the estrogen receptor (ER), and is a full antagonist for the MR which is beneficial during pregnancy; counteracting possibly excess water retention induced by estrogens (29,30,31) [6].
P4 is slightly anti-androgenic because it also binds to 5-α reductase enzyme and will therefore interact with the conversion of testosterone in dihydrotestosterone, its active metabolite (2) [6]. This is analogous to dutasteride and finasteride.
Safety and Pharmacodynamics in men
There are no established safety profiles for progesterone in men [6]. Safety profiles in women differ depending on when these hormones are used during the menstrual cycle, in early and late pregnancy and in the alleviation of peri- or postmenopausal symptoms [6].
Trying to divine the pharmacodynamics of these exogenous classical female hormones in men should likely focus on the activity on the CNS and on activity in binding the classical nuclear steroid family, chiefly the progesterone receptor (PR), androgen receptor (AR), and mineralocorticoid receptor (MR). Generally, progestagens and (synthetic) progestins agonize the PR, antagonize the AR, agonize the GR, and antagonize the MR.
These classical female hormones regulate the menstrual cycle and pregnancy, and thus certainly have myriad unquantifiable effects in the male organism. Progesterone produced in the luteal phase of the menstrual cycle has several physiological effects regulating menses, and in the pregnant uterus, controlling the development of endometrial receptivity preparing the endometrium for implantation [6].
It is difficult to assess the full spectrum of genomic and nongenomic action of these hormones in men given the paucity of research on these hormones in this population.
Progesterone and its metabolites (allopregnanolone and 4α,5α-tetrahydrodeoxycorticosterone) are potent activators of the PR. Extra-nuclear, non-classical (nongenomic) effects mechanisms include interactions with membrane receptors from the oxytocin (the "bonding" hormone) and GABA-A receptors, and the induction of a direct relaxing effect on uterine contractility by blocking calcium influx [6].
Activation of the PR regulates mammalian female sexual behavior (heat, behavioral estrus), and concurrent treatment with E2 (estradiol) treatment with E2 maximizes the probability that the female will display “lordosis” response, a primary reflexive component of female reproductive behavior, upon mounting by a con-specific male [11].
The extent of activity of progesterone on the CNS is modulated by the route of administration: oral P4 is affected by the presence of bacteria and gut enzymes, the intestinal wall, and liver, wheras intramuscular P4 is not [6].
Micronized progesterone (P4) and allopregnanolone, its chief metabolite, modulate GABAergic transmission with a similar potency or even greater efficacy than alcohol, benzodiazepines, or barbiturates [6]. Thus, similar to mesterolone (Proviron), this hormone is likely to be accompanied by withdrawal symptoms and there is no reason to believe that it is not reinforcing (add
ictive), as a class effect of GABAergic agents.
Oral route
Orally administered P4 undergoes several successive metabolic steps in the gut (5β-reductase), in the intestinal wall (5α-reductase), and the liver (5β-reductase, 3α- & 20α- hydroxylase). The resulting metabolites, 5α-pregnanolone (allopregnanolne; AlloP) and 5β-pregnanolone, bind the GABA-A-R, whilst 5α-pregnanedione & 5β-pregnanedione exert anti-mitotic (suppressing growth) and tocolytic (anti-contraction/relaxation) effects [6]. Oral P4 increases bone formation and is attended by estrogen-related improvements in bone mineral density (BMD), likely via production of new osteoblasts from mesenchymal stem cells and stimulation of osteoblasts to generate bone matrix (8),,[6],,. Oral P4 results in rapid absorption, a maximal plasma concentration within 4 hr with a 8.6% bioavailability versus intramuscular administration, and twice that when administered before food (5) [6].
Intramuscular route
Analogous to the most common (vaginal) route of administration, the intramuscular (IM) route of P4 results in only a small increase in allopregnanolone and no change in 5β-pregnanolone. Thus, men seeking the most relevant function (GABA-A-R modulation) of this hormone would be advised to use via the oral route (and, this is likely to modulate serum levels within the endogenous male range.
Reduced HPG Axis Functioning
"HPTA suppression"
Progesterone and its derivatives dysregulate hypothalamic regulation of T and gonadotropins via KNDy dendron signalling, disrupting GnRH pulsatility, and inhibiting pituitary LH secretion [8] [9]. Synthetic progestins used in male contraception derive efficacy from this feature. Bebb, et al. randomized healthy men to receive either testosterone enanthate (100 mg weekly), or the same dosage of testosterone in combination with the progestin levonorgestrel, the addition of which virtually abolished LH and FSH secretion [10].
Circulating levels in healthy adult men
AlloP serum:
- 0.75 nmol/L
- 0.24 ng/mL
Prog serum:
- 1.9 nmol/L
- 0.60 ng/mL
DHEA serum:
- 16.33 nmol/L
- 4.71 ng/mL [3]
DHEA is primarily an adrenal steroid; AlloP and Prog, as active neurosteroids, are primarily synthesized in the brain.
If one does embark on the use of exogenous neuroactive steroids, it would be prudent to "dial in" bloodwork to healthy endogenous male serum levels.
Whether the adult male's interest in
- allopregnanolone and/or
- exogenous (e.g., micronized) progesterone (P4)
- to treat:
+ anxiety (with drug-like efficacy; on par or greater than benzodiazepines or barbiturates, alcohol)
+ depression (with drug-like efficacy)
+ bones/joints (potential bone formation; augmented BMD)
+ adverse sexual effects of the rare (up to 4% prevalence) post-finasteride syndrome (the efficacy for which these agents have never been rigorously examined scientifically)
- outweighs:
- negative impact on HPG axis functioning (HPTA)
- anti-androgenic action (indeed, progesterone functions analogously to dutasteride/finasteride)
- potential for habituation (addiction) as GABAergic agents
- off-target hormonal effects (particularly egregious with progesterone)
- in consideration of:
+ exogenous T supplanting the role of DHT (but not allopregnanolone, progesterone [whose function in male physiology is markedly limited]) in prostate, scalp, etc.
+ exogenous T potently stimulating osteoblast activity (bone formation; augmented BMD)
+ availability of clinically indicated treatments, including TRT which demonstrably improves its patients' mental health, sexual function, QoL, and bone mineral density, administered by medical professionals, and
- the absence of any demonstrable clinical relevance of supplemental neurosteroids for healthy men
_______________________________
References:
[1] Zorumski, C. F., Paul, S. M., Covey, D. F., & Mennerick, S. (2019). Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiology of Stress, 11, 100196. doi:10.1016/j.ynstr.2019.100196
[2] Irwig, M. S. (2014). Persistent Sexual and Nonsexual Adverse Effects of Finasteride in Younger Men. Sexual Medicine Reviews, 2(1), 24–35. doi:10.1002/smrj.19
[3] Genazzani, A. R., Petraglia, F., Bernardi, F., Casarosa, E., Salvestroni, C., Tonetti, A., … Luisi, M. (1998). Circulating Levels of Allopregnanolone in Humans: Gender, Age, and Endocrine Influences. The Journal of Clinical Endocrinology & Metabolism, 83(6), 2099–2103. doi:10.1210/jcem.83.6.4905
[4] Bond, P. (2020). On Steroids.
[5] Schumacher, M., Mattern, C., Ghoumari, A., Oudinet, J. P., Liere, P., Labombarda, F., … Guennoun, R. (2014). Revisiting the roles of progesterone and allopregnanolone in the nervous system: Resurgence of the progesterone receptors. Progress in Neurobiology, 113, 6–39. doi:10.1016/j.pneurobio.2013.09.004
[6] Piette, P. C. (2020). The Pharmacodynamics and Safety of Progesterone. Best Practice & Research Clinical Obstetrics & Gynaecology. doi:10.1016/j.bpobgyn.2020.06.002
[7] Sitruk-Ware, R. (2004). Pharmacological profile of progestins. Maturitas, 47(4), 277–283. doi:10.1016/j.maturitas.2004.01.001
[8] Navarro, V. M., Gottsch, M. L., Chavkin, C., Okamura, H., Clifton, D. K., & Steiner, R. A. (2009). Regulation of Gonadotropin-Releasing Hormone Secretion by Kisspeptin/Dynorphin/Neurokinin B Neurons in the Arcuate Nucleus of the Mouse. Journal of Neuroscience, 29(38), 11859–11866. doi:10.1523/jneurosci.1569-09.2009
[9] Girmus, R. L., & Wise, M. E. (1992). Progesterone Directly Inhibits Pituitary Luteinizing Hormone Secretion in an Estradiol-dependent Manner1. Biology of Reproduction, 46(4), 710–714. doi:10.1095/biolreprod46.4.710
[10] Bebb, R. A., Anawalt, B. D., Christensen, R. B., Paulsen, C. A., Bremner, W. J., & Matsumoto, A. M. (1996). Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: a promising male contraceptive approach. The Journal of Clinical Endocrinology & Metabolism, 81(2), 757–762. doi:10.1210/jcem.81.2.8636300
[11] Mani S, Portillo W. Activation of progestin receptors in female reproductive behavior: Interactions with neurotransmitters. Front Neuroendocrinol. 2010;31(2):157-171. doi:10.1016/j.yfrne.2010.01.002
Author: Type-IIx
It has come to my attention that there is a growing number of bodybuilders and AAS users subscribing to the practice of administering exogenous progesterone or allopregnanolone (or both) as supplemental neurosteroids. I want to put this information out there for them, so that they may balance the tradeoffs of this risky endeavour with appropriate information from different sources.
This article purposely discusses progestins (synthetic) and progesterone receptor agonists, including "bioidentical" progesterone (which is not inherently safe due to its being "bioidentical...") as a singular class of agents that are classic female hormonal agents. A later "article" will be forthcoming that distinguishes between progestins, progesterone, and prolactin (as there is widespread confusion, understandably).
Neurosteroids: steroids that are synthesized in the CNS from cholesterol or sterol precursors [1].

Biosynthesis of allopregnanolone [5].
Allopregnanolone
[1]The recent FDA approval of the neurosteroid, brexanolone (allopregnanolone), as a treatment for women with postpartum depression, and successful trials of a related neuroactive steroid, SGE-217, for men and women with major depressive disorder offer the hope of a new era in treating mood and anxiety disorders based on the potential of neurosteroids as modulators of brain function... contributing to antidepressant and anxiolytic effects of allopregnanolone and other GABAergic neurosteroids ... as positive allosteric modulators of GABA-A receptors. We also consider their roles as endogenous "stress" modulators and possible additional mechanisms contributing to their therapeutic effects. We argue that further understanding of the molecular, cellular, network and psychiatric effects of neurosteroids offers the hope of further advances in the treatment of mood and anxiety disorders.
Allopregnanolone: potent and positive allosteric modulator (PAM) of GABA-A receptors [1], regulates stress, mood, and female sexual behavior [3].
Allopregnanolone exhibits benzodiazepine and antidepressant qualities by modulating GABA-A receptors. Allopregnanolone and progesterone, as steroids, are broad spectrum in their actions, meaning that they affect multiple systems in unpredictable ways.
Progesterone
The "mother molecule"
[4]Progesterone is a steroid hormone, and, like prolactin, it seems to largely play an irrelevant role in male physiology. Again, don't misunderstand my statement. I'm not saying it does nothing. It's an important precursor to the endogenous production of some other steroid hormones (aldosterone and cortisol). Additionally, it affects the brain [5].
Progesterone and its metabolites are neurosteroids that also play an important role in myelination. Consequently, blocking the production of the metabolites of progesterone has been found to have an adverse effect on the myelination process. In rodent and in vitro models, there is evidence of therapeutic benefits for certain types of injury (ischemic stroke, ALS, MS, carpal tunnel syndrome) (22) [6]. Progesterone is upregulated in TBI and stroke patients.
Thus, exogenous progesterone likely has some efficacy in recovery from TBI and stroke, and perhaps for demyelinating disorders. It also has demonstrable efficacy in the treatment of postpartum depression.
Micronized progesterone
Micronised P4 (but not synthetic progestins) and one of its major metabolites, allopregnanolone, have been shown to modulate GABAergic transmission with a similar potency or even greater efficacy, than those of alcohol, benzodiazepines, or barbiturates (4) [6].
P4 is a weak agonist for the GR and AR, has no significant activity via the estrogen receptor (ER), and is a full antagonist for the MR which is beneficial during pregnancy; counteracting possibly excess water retention induced by estrogens (29,30,31) [6].
P4 is slightly anti-androgenic because it also binds to 5-α reductase enzyme and will therefore interact with the conversion of testosterone in dihydrotestosterone, its active metabolite (2) [6]. This is analogous to dutasteride and finasteride.
Safety and Pharmacodynamics in men
There are no established safety profiles for progesterone in men [6]. Safety profiles in women differ depending on when these hormones are used during the menstrual cycle, in early and late pregnancy and in the alleviation of peri- or postmenopausal symptoms [6].
Trying to divine the pharmacodynamics of these exogenous classical female hormones in men should likely focus on the activity on the CNS and on activity in binding the classical nuclear steroid family, chiefly the progesterone receptor (PR), androgen receptor (AR), and mineralocorticoid receptor (MR). Generally, progestagens and (synthetic) progestins agonize the PR, antagonize the AR, agonize the GR, and antagonize the MR.
These classical female hormones regulate the menstrual cycle and pregnancy, and thus certainly have myriad unquantifiable effects in the male organism. Progesterone produced in the luteal phase of the menstrual cycle has several physiological effects regulating menses, and in the pregnant uterus, controlling the development of endometrial receptivity preparing the endometrium for implantation [6].
It is difficult to assess the full spectrum of genomic and nongenomic action of these hormones in men given the paucity of research on these hormones in this population.
Progesterone and its metabolites (allopregnanolone and 4α,5α-tetrahydrodeoxycorticosterone) are potent activators of the PR. Extra-nuclear, non-classical (nongenomic) effects mechanisms include interactions with membrane receptors from the oxytocin (the "bonding" hormone) and GABA-A receptors, and the induction of a direct relaxing effect on uterine contractility by blocking calcium influx [6].
Activation of the PR regulates mammalian female sexual behavior (heat, behavioral estrus), and concurrent treatment with E2 (estradiol) treatment with E2 maximizes the probability that the female will display “lordosis” response, a primary reflexive component of female reproductive behavior, upon mounting by a con-specific male [11].
The extent of activity of progesterone on the CNS is modulated by the route of administration: oral P4 is affected by the presence of bacteria and gut enzymes, the intestinal wall, and liver, wheras intramuscular P4 is not [6].
Micronized progesterone (P4) and allopregnanolone, its chief metabolite, modulate GABAergic transmission with a similar potency or even greater efficacy than alcohol, benzodiazepines, or barbiturates [6]. Thus, similar to mesterolone (Proviron), this hormone is likely to be accompanied by withdrawal symptoms and there is no reason to believe that it is not reinforcing (add
ictive), as a class effect of GABAergic agents.
Oral route
Orally administered P4 undergoes several successive metabolic steps in the gut (5β-reductase), in the intestinal wall (5α-reductase), and the liver (5β-reductase, 3α- & 20α- hydroxylase). The resulting metabolites, 5α-pregnanolone (allopregnanolne; AlloP) and 5β-pregnanolone, bind the GABA-A-R, whilst 5α-pregnanedione & 5β-pregnanedione exert anti-mitotic (suppressing growth) and tocolytic (anti-contraction/relaxation) effects [6]. Oral P4 increases bone formation and is attended by estrogen-related improvements in bone mineral density (BMD), likely via production of new osteoblasts from mesenchymal stem cells and stimulation of osteoblasts to generate bone matrix (8),,[6],,. Oral P4 results in rapid absorption, a maximal plasma concentration within 4 hr with a 8.6% bioavailability versus intramuscular administration, and twice that when administered before food (5) [6].
Intramuscular route
Analogous to the most common (vaginal) route of administration, the intramuscular (IM) route of P4 results in only a small increase in allopregnanolone and no change in 5β-pregnanolone. Thus, men seeking the most relevant function (GABA-A-R modulation) of this hormone would be advised to use via the oral route (and, this is likely to modulate serum levels within the endogenous male range.
Reduced HPG Axis Functioning
"HPTA suppression"
Progesterone and its derivatives dysregulate hypothalamic regulation of T and gonadotropins via KNDy dendron signalling, disrupting GnRH pulsatility, and inhibiting pituitary LH secretion [8] [9]. Synthetic progestins used in male contraception derive efficacy from this feature. Bebb, et al. randomized healthy men to receive either testosterone enanthate (100 mg weekly), or the same dosage of testosterone in combination with the progestin levonorgestrel, the addition of which virtually abolished LH and FSH secretion [10].
The effects of progestins relate to their interactions with receptors: AR (e.g., acnea, lipid effects); glucocorticoid receptors (GR) (eg., salt and water retention, bloating); or mineralocorticoid receptors (e.g., decreased water retention and weight). Anti-androgenic progestins may act in several ways. They can exert competitive inhibition of the AR, or bind to the enzyme 5-alpha reductase and hence interact with the conversion of testosterone into dihydrotestosterone (its active metabolite). When combined with estrogen the non-androgenic progestins do not oppose the estrogen-dependent increase in SHBG. The latter effect results in more binding of the circulating androgens and less free T available for action at the receptor level. Thus, anti-androgenic progestins may have beneficial effects (e.g., controlling endogenous androgen and decreasing acnea or hirsutism) [7].
Circulating levels in healthy adult men
AlloP serum:
- 0.75 nmol/L
- 0.24 ng/mL
Prog serum:
- 1.9 nmol/L
- 0.60 ng/mL
DHEA serum:
- 16.33 nmol/L
- 4.71 ng/mL [3]
DHEA is primarily an adrenal steroid; AlloP and Prog, as active neurosteroids, are primarily synthesized in the brain.
If one does embark on the use of exogenous neuroactive steroids, it would be prudent to "dial in" bloodwork to healthy endogenous male serum levels.
Whether the adult male's interest in
- allopregnanolone and/or
- exogenous (e.g., micronized) progesterone (P4)
- to treat:
+ anxiety (with drug-like efficacy; on par or greater than benzodiazepines or barbiturates, alcohol)
+ depression (with drug-like efficacy)
+ bones/joints (potential bone formation; augmented BMD)
+ adverse sexual effects of the rare (up to 4% prevalence) post-finasteride syndrome (the efficacy for which these agents have never been rigorously examined scientifically)
- outweighs:
- negative impact on HPG axis functioning (HPTA)
- anti-androgenic action (indeed, progesterone functions analogously to dutasteride/finasteride)
- potential for habituation (addiction) as GABAergic agents
- off-target hormonal effects (particularly egregious with progesterone)
- in consideration of:
+ exogenous T supplanting the role of DHT (but not allopregnanolone, progesterone [whose function in male physiology is markedly limited]) in prostate, scalp, etc.
+ exogenous T potently stimulating osteoblast activity (bone formation; augmented BMD)
+ availability of clinically indicated treatments, including TRT which demonstrably improves its patients' mental health, sexual function, QoL, and bone mineral density, administered by medical professionals, and
- the absence of any demonstrable clinical relevance of supplemental neurosteroids for healthy men
_______________________________
References:
[1] Zorumski, C. F., Paul, S. M., Covey, D. F., & Mennerick, S. (2019). Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiology of Stress, 11, 100196. doi:10.1016/j.ynstr.2019.100196
[2] Irwig, M. S. (2014). Persistent Sexual and Nonsexual Adverse Effects of Finasteride in Younger Men. Sexual Medicine Reviews, 2(1), 24–35. doi:10.1002/smrj.19
[3] Genazzani, A. R., Petraglia, F., Bernardi, F., Casarosa, E., Salvestroni, C., Tonetti, A., … Luisi, M. (1998). Circulating Levels of Allopregnanolone in Humans: Gender, Age, and Endocrine Influences. The Journal of Clinical Endocrinology & Metabolism, 83(6), 2099–2103. doi:10.1210/jcem.83.6.4905
[4] Bond, P. (2020). On Steroids.
[5] Schumacher, M., Mattern, C., Ghoumari, A., Oudinet, J. P., Liere, P., Labombarda, F., … Guennoun, R. (2014). Revisiting the roles of progesterone and allopregnanolone in the nervous system: Resurgence of the progesterone receptors. Progress in Neurobiology, 113, 6–39. doi:10.1016/j.pneurobio.2013.09.004
[6] Piette, P. C. (2020). The Pharmacodynamics and Safety of Progesterone. Best Practice & Research Clinical Obstetrics & Gynaecology. doi:10.1016/j.bpobgyn.2020.06.002
[7] Sitruk-Ware, R. (2004). Pharmacological profile of progestins. Maturitas, 47(4), 277–283. doi:10.1016/j.maturitas.2004.01.001
[8] Navarro, V. M., Gottsch, M. L., Chavkin, C., Okamura, H., Clifton, D. K., & Steiner, R. A. (2009). Regulation of Gonadotropin-Releasing Hormone Secretion by Kisspeptin/Dynorphin/Neurokinin B Neurons in the Arcuate Nucleus of the Mouse. Journal of Neuroscience, 29(38), 11859–11866. doi:10.1523/jneurosci.1569-09.2009
[9] Girmus, R. L., & Wise, M. E. (1992). Progesterone Directly Inhibits Pituitary Luteinizing Hormone Secretion in an Estradiol-dependent Manner1. Biology of Reproduction, 46(4), 710–714. doi:10.1095/biolreprod46.4.710
[10] Bebb, R. A., Anawalt, B. D., Christensen, R. B., Paulsen, C. A., Bremner, W. J., & Matsumoto, A. M. (1996). Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: a promising male contraceptive approach. The Journal of Clinical Endocrinology & Metabolism, 81(2), 757–762. doi:10.1210/jcem.81.2.8636300
[11] Mani S, Portillo W. Activation of progestin receptors in female reproductive behavior: Interactions with neurotransmitters. Front Neuroendocrinol. 2010;31(2):157-171. doi:10.1016/j.yfrne.2010.01.002