Role of Hypothalamus in Aging and Its Underlying Cellular Mechanisms
Highlights
· The hypothalamic aging is critical for systemic aging.
· Functional changes in a group of the hypothalamic neurons contribute to age-associated decline in energy homeostasis, hormone balance, circadian rhythm, and reproduction.
· The underlying cellular mechanism for hypothalamus-mediated aging includes nutrient sensing dysfunction, altered intercellular communication, hypothalamic stem cell exhaustion, proteostasis loss, and epigenetic alterations.
· Further dissection of hypothalamic molecular signature of aging will be instrumental in understanding systemic aging and developing therapeutic intervention for the extension of healthy lifespan.
Aging is characterized by a progressive loss of several physiological functions that can cause various age-related disorders. Several factors have been identified as causes of aging to elucidate the decline in functions. Various aspects of physiological deterioration are controlled by the hypothalamus, a critical brain region that connects the neuroendocrine system to physiological functions.
In addition, functional alterations in a set of agouti-related peptide/neuropeptide Y (AgRP/NPY) and pro-opiomelanocortin (POMC) neurons, a set of growth hormone-releasing hormone (GHRH) and somatostatin (SST) neurons, a set of arginine vasopressin (AVP) and vasoactive intestinal peptide (VIP) neurons, and a set of gonadotropin-releasing hormone (GnRH) and kisspeptin/neurokinin B/dynorphin (KNDy) neurons contribute to age-related physiological decline in energy metabolism, hormone regulation, circadian rhythm, and reproduction, respectively.
The underlying cellular mechanism for the hypothalamus-mediated aging progression comprises dysregulation of nutrient sensing, altered intercellular communication, stem cell exhaustion, loss of proteostasis, and epigenetic alterations. Furthermore, mammalian target of rapamycin (mTOR), NF-kB, hypothalamic stem cell, autophagy, and SIRT1 have been recognized as critical factors or pathways mediating the mechanism. Perhaps, further dissection of these pathways or components could provide the potential for developing a therapeutic intervention for age-related diseases or the extension of healthy lifespan.
Kim K, Choe HK. Role of hypothalamus in aging and its underlying cellular mechanisms. Mechanisms of Ageing and Development. Role of hypothalamus in aging and its underlying cellular mechanisms
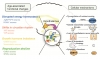
The Hypothalamus As A Regulator Of Systemic Aging.
We propose a working model that the hypothalamus controls several aspects of systemic aging.
Here, an age-dependent decline in physiological functions, including disruption of energy homeostasis, shifts in the circadian rhythm, imbalance in GH levels, and decline in reproduction, is mediated through age-associated changes in the master regulatory neurons, such as the AgRP/NPY, POMC, AVP, VIP, GHRH, SST, GnRH, and KNDy neurons.
Notably, the hypothalamus is also a region where a majority of molecular pathways implicated in aging, such as nutrient sensing, inflammation, neural stem cell, proteostasis, and epigenetic regulation, are altered with aging.
Highlights
· The hypothalamic aging is critical for systemic aging.
· Functional changes in a group of the hypothalamic neurons contribute to age-associated decline in energy homeostasis, hormone balance, circadian rhythm, and reproduction.
· The underlying cellular mechanism for hypothalamus-mediated aging includes nutrient sensing dysfunction, altered intercellular communication, hypothalamic stem cell exhaustion, proteostasis loss, and epigenetic alterations.
· Further dissection of hypothalamic molecular signature of aging will be instrumental in understanding systemic aging and developing therapeutic intervention for the extension of healthy lifespan.
Aging is characterized by a progressive loss of several physiological functions that can cause various age-related disorders. Several factors have been identified as causes of aging to elucidate the decline in functions. Various aspects of physiological deterioration are controlled by the hypothalamus, a critical brain region that connects the neuroendocrine system to physiological functions.
In addition, functional alterations in a set of agouti-related peptide/neuropeptide Y (AgRP/NPY) and pro-opiomelanocortin (POMC) neurons, a set of growth hormone-releasing hormone (GHRH) and somatostatin (SST) neurons, a set of arginine vasopressin (AVP) and vasoactive intestinal peptide (VIP) neurons, and a set of gonadotropin-releasing hormone (GnRH) and kisspeptin/neurokinin B/dynorphin (KNDy) neurons contribute to age-related physiological decline in energy metabolism, hormone regulation, circadian rhythm, and reproduction, respectively.
The underlying cellular mechanism for the hypothalamus-mediated aging progression comprises dysregulation of nutrient sensing, altered intercellular communication, stem cell exhaustion, loss of proteostasis, and epigenetic alterations. Furthermore, mammalian target of rapamycin (mTOR), NF-kB, hypothalamic stem cell, autophagy, and SIRT1 have been recognized as critical factors or pathways mediating the mechanism. Perhaps, further dissection of these pathways or components could provide the potential for developing a therapeutic intervention for age-related diseases or the extension of healthy lifespan.
Kim K, Choe HK. Role of hypothalamus in aging and its underlying cellular mechanisms. Mechanisms of Ageing and Development. Role of hypothalamus in aging and its underlying cellular mechanisms
The Hypothalamus As A Regulator Of Systemic Aging.
We propose a working model that the hypothalamus controls several aspects of systemic aging.
Here, an age-dependent decline in physiological functions, including disruption of energy homeostasis, shifts in the circadian rhythm, imbalance in GH levels, and decline in reproduction, is mediated through age-associated changes in the master regulatory neurons, such as the AgRP/NPY, POMC, AVP, VIP, GHRH, SST, GnRH, and KNDy neurons.
Notably, the hypothalamus is also a region where a majority of molecular pathways implicated in aging, such as nutrient sensing, inflammation, neural stem cell, proteostasis, and epigenetic regulation, are altered with aging.


