Vega A, Martinot E, Baptissart M, et al. Identification of the link between the hypothalamo-pituitary axis and the testicular orphan nuclear receptor NR0B2 in adult male mice. Endocrinology. http://press.endocrine.org/doi/abs/10.1210/en.2014-1418
The small heterodimer partner (NR0B2) is an atypical nuclear receptor known mainly for its role in bile acid homeostasis in the enterohepatic tract. We previously showed that NR0B2 controls testicular functions such as testosterone synthesis. Moreover, NR0B2 mediates the deleterious testicular effects of estrogenic endocrine disruptors leading to infertility.
The endocrine homeostasis is essential for health as it controls many physiological functions. This is supported by a large number of studies demonstrating that alterations of steroid activity lead to several kinds of diseases such as obesity and infertility.
Within the testis, the functions of the Leydig cells are mainly controlled by the hypothalamo-pituitary (HP) axis via luteinizing hormone/chorionic gonadotropin (LH/CG). Here we show that LH/CG represses Nr0b2 expression through the PKA-AMPK pathway.
Moreover, using a transgenic mouse model invalidated for Nr0b2, we point out that NR0B2 mediates the repression of testosterone synthesis and subsequent germ cell apoptosis induced by exposure to anti-GnRH compound.
Together, our data demonstrate a new link between HP axis and NR0B2 in testicular androgen metabolism, making NR0B2 a major actor of testicular physiology in case of alteration of LH/CG levels.
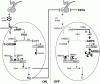
Role of NR0B2 in the Control of Testicular Steroidogenesis, A Proposed Model
When LH is secreted, cAMP-PKA pathway is activated and the system is “ON”.
P-CREB, NR5A1 and NR5A1 will transcriptionally regulate StAR expression. At the same time, Nr0b2 expression is maintained low via the inhibition of AMPK pathway.
T and E2 increase will induce a negative feedback on LH secretion.
Production of AMP by phosphodiesterases (PDEs) and decreased activity of cAMP-PKA pathway resulted in an increase of P-AMPK levels leading to NR0B2 accumulation, which in turn, through its interaction with NR5A1 and NR5A2, will reduce Star expression and steroid production.
[Note: PDE5i (Viagra) increase testosterone synthesis.]
In pathophysiological condition, this low LH/CG levels could be observed in the context of estrogenic endocrine disrupters (EEDs) exposure.
Indeed, exposure to EEDs results in a lower LH secretion and thus can induce testicular deleterious effects via the same signaling pathway involving NR0B2.
The small heterodimer partner (NR0B2) is an atypical nuclear receptor known mainly for its role in bile acid homeostasis in the enterohepatic tract. We previously showed that NR0B2 controls testicular functions such as testosterone synthesis. Moreover, NR0B2 mediates the deleterious testicular effects of estrogenic endocrine disruptors leading to infertility.
The endocrine homeostasis is essential for health as it controls many physiological functions. This is supported by a large number of studies demonstrating that alterations of steroid activity lead to several kinds of diseases such as obesity and infertility.
Within the testis, the functions of the Leydig cells are mainly controlled by the hypothalamo-pituitary (HP) axis via luteinizing hormone/chorionic gonadotropin (LH/CG). Here we show that LH/CG represses Nr0b2 expression through the PKA-AMPK pathway.
Moreover, using a transgenic mouse model invalidated for Nr0b2, we point out that NR0B2 mediates the repression of testosterone synthesis and subsequent germ cell apoptosis induced by exposure to anti-GnRH compound.
Together, our data demonstrate a new link between HP axis and NR0B2 in testicular androgen metabolism, making NR0B2 a major actor of testicular physiology in case of alteration of LH/CG levels.
Role of NR0B2 in the Control of Testicular Steroidogenesis, A Proposed Model
When LH is secreted, cAMP-PKA pathway is activated and the system is “ON”.
P-CREB, NR5A1 and NR5A1 will transcriptionally regulate StAR expression. At the same time, Nr0b2 expression is maintained low via the inhibition of AMPK pathway.
T and E2 increase will induce a negative feedback on LH secretion.
Production of AMP by phosphodiesterases (PDEs) and decreased activity of cAMP-PKA pathway resulted in an increase of P-AMPK levels leading to NR0B2 accumulation, which in turn, through its interaction with NR5A1 and NR5A2, will reduce Star expression and steroid production.
[Note: PDE5i (Viagra) increase testosterone synthesis.]
In pathophysiological condition, this low LH/CG levels could be observed in the context of estrogenic endocrine disrupters (EEDs) exposure.
Indeed, exposure to EEDs results in a lower LH secretion and thus can induce testicular deleterious effects via the same signaling pathway involving NR0B2.


