Re: OnLine First
Model For The Mechanism Of The Anabolic Action Of Steroid Hormones
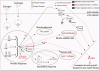
At a time when the increase in muscle mass had already become apparent, researchers interrogated high-throughput gene expression data from muscle samples using two recently published co-expression based network inference approaches called Partial Correlation coefficient with Information Theory (PCIT) and Regulatory Impact Factor (RIF), and the promoter structure of the differentially expressed genes. Their results point to a dramatic and unexpected induction of muscle oxytocin (OXT) expression also measurable in the plasma at the protein level. They hypothesise that the increased expression of OXT may account for the increased muscle mass.
Estrogen and androgen bind to intracellular receptors, which in turn bind to promoter regions of specific genes to either induce or repress expression of the primary response to steroid treatment. Muscle is composed of a mixture of cell types, which are likely to respond differently to the exposure to steroids.
As part of the response, a group of fat metabolism genes is down-regulated via an unknown mechanism, which may simply reflect a decrease in the lipid storage activity of intramuscular adipocytes due to the increased bio-energetic demand of supporting enhanced muscle growth. Also as part of the response, a group of genes encoding collagen subunits is up-regulated by an unknown mechanism, probably in the mature muscle cells. It is most likely that the increase in cell cycle gene expression occurs in the muscle satellite cells, as differentiated muscle cells do not divide and the activity of adipocytes storing lipid is decreased, which is not consistent with increased cell numbers.
The small increase in expression of IGF1 is consistent with IGF1 playing a role in the increase in muscle growth. However, the Regulatory Impact analysis (RIF) analysis more strongly supports the oxytocin receptor, OXTR, than the IGF1 receptor IGF1R, as a key driver in the phenotype induced by the steroid treatment. The signalling cascade downstream of the receptor remains somewhat enigmatic. Based on the experiments described here a much more comprehensive and more detailed picture is now available of the mechanisms by which the steroid treatment leads to the observed phenotypes of increased average daily gain.
Future studies should evaluate the association between increased OXT levels and other traits related to Hormone Growth Promotant (HGP) treatment including increased bone density, increased meat toughness and reduced intramuscular fat content. It is likely that these mechanisms will be at least partially applicable across the mammals. There are still many gaps in our understanding, not the least of which is, does oxytocin alone have an anabolic activity?

Schematic diagram of a possible mechanism whereby the sex-steroid hormones trenbolone acetate and estradiol could drive skeletal muscle growth. The solid lines represent direct actions for which the authors have supporting evidence and the broken lines represent either indirect actions or incomplete evidence. Double headed arrows indicate known protein-protein interactions.
De Jager N, Hudson NJ, Reverter A, et al. Chronic exposure to anabolic steroids induces the muscle expression of oxytocin and a more than fifty fold increase in circulating oxytocin in cattle. Physiol Genomics. http://physiolgenomics.physiology.org/content/early/2011/02/15/physiolgenomics.00226.2010.abstract
Molecular mechanisms in skeletal muscle associated with anabolic steroid treatment of cattle are unclear and we aimed to characterise transcriptional changes. Cattle were chronically exposed (68 ± 20 days) to a steroid hormone implant containing mg trenbolone acetate and 20mg estradiol (Revalor-H). Biopsy samples from 48 cattle (half treated) from Longissimus dorsi muscle (LD) under local anaesthesia were collected and gene expression levels were profiled by microarray, covering 44 unique bovine genes. One hundred and twenty one genes were differentially expressed (DE) due to the implant (99.99% posterior probability of not being false positives). Among DE genes, a decrease in expression of a number of fat metabolism associated genes, likely reflecting the lipid storage activity of intramuscular adipocytes, was observed. The expression of IGF1 and genes related to the extra-cellular matrix, slow twitch fibres and cell cycle (including SOX8, a satellite cell marker) was increased in the treated muscle. Unexpectedly, a very large 21- (microarray) to 97- (real time quantitative PCR) fold higher expression of the mRNA encoding the neuropeptide hormone oxytocin was observed in treated muscle, We also observed an ~50-fold higher level of circulating oxytocin in the plasma of treated animals at the time of biopsy. Using a co-expression network strategy OXTR was identified as more likely than IGF1R to be a major mediator of the muscle response to Revalor-H. A re-investigation of in vivo cattle LD muscle samples during early to mid-fetal development identified a > fold increased expression of OXT, coincident with myofibre differentiation and fusion. We propose that oxytocin may be involved in mediating the anabolic effects of Revalor-H treatment.
Model For The Mechanism Of The Anabolic Action Of Steroid Hormones
At a time when the increase in muscle mass had already become apparent, researchers interrogated high-throughput gene expression data from muscle samples using two recently published co-expression based network inference approaches called Partial Correlation coefficient with Information Theory (PCIT) and Regulatory Impact Factor (RIF), and the promoter structure of the differentially expressed genes. Their results point to a dramatic and unexpected induction of muscle oxytocin (OXT) expression also measurable in the plasma at the protein level. They hypothesise that the increased expression of OXT may account for the increased muscle mass.
Estrogen and androgen bind to intracellular receptors, which in turn bind to promoter regions of specific genes to either induce or repress expression of the primary response to steroid treatment. Muscle is composed of a mixture of cell types, which are likely to respond differently to the exposure to steroids.
As part of the response, a group of fat metabolism genes is down-regulated via an unknown mechanism, which may simply reflect a decrease in the lipid storage activity of intramuscular adipocytes due to the increased bio-energetic demand of supporting enhanced muscle growth. Also as part of the response, a group of genes encoding collagen subunits is up-regulated by an unknown mechanism, probably in the mature muscle cells. It is most likely that the increase in cell cycle gene expression occurs in the muscle satellite cells, as differentiated muscle cells do not divide and the activity of adipocytes storing lipid is decreased, which is not consistent with increased cell numbers.
The small increase in expression of IGF1 is consistent with IGF1 playing a role in the increase in muscle growth. However, the Regulatory Impact analysis (RIF) analysis more strongly supports the oxytocin receptor, OXTR, than the IGF1 receptor IGF1R, as a key driver in the phenotype induced by the steroid treatment. The signalling cascade downstream of the receptor remains somewhat enigmatic. Based on the experiments described here a much more comprehensive and more detailed picture is now available of the mechanisms by which the steroid treatment leads to the observed phenotypes of increased average daily gain.
Future studies should evaluate the association between increased OXT levels and other traits related to Hormone Growth Promotant (HGP) treatment including increased bone density, increased meat toughness and reduced intramuscular fat content. It is likely that these mechanisms will be at least partially applicable across the mammals. There are still many gaps in our understanding, not the least of which is, does oxytocin alone have an anabolic activity?
Schematic diagram of a possible mechanism whereby the sex-steroid hormones trenbolone acetate and estradiol could drive skeletal muscle growth. The solid lines represent direct actions for which the authors have supporting evidence and the broken lines represent either indirect actions or incomplete evidence. Double headed arrows indicate known protein-protein interactions.
De Jager N, Hudson NJ, Reverter A, et al. Chronic exposure to anabolic steroids induces the muscle expression of oxytocin and a more than fifty fold increase in circulating oxytocin in cattle. Physiol Genomics. http://physiolgenomics.physiology.org/content/early/2011/02/15/physiolgenomics.00226.2010.abstract
Molecular mechanisms in skeletal muscle associated with anabolic steroid treatment of cattle are unclear and we aimed to characterise transcriptional changes. Cattle were chronically exposed (68 ± 20 days) to a steroid hormone implant containing mg trenbolone acetate and 20mg estradiol (Revalor-H). Biopsy samples from 48 cattle (half treated) from Longissimus dorsi muscle (LD) under local anaesthesia were collected and gene expression levels were profiled by microarray, covering 44 unique bovine genes. One hundred and twenty one genes were differentially expressed (DE) due to the implant (99.99% posterior probability of not being false positives). Among DE genes, a decrease in expression of a number of fat metabolism associated genes, likely reflecting the lipid storage activity of intramuscular adipocytes, was observed. The expression of IGF1 and genes related to the extra-cellular matrix, slow twitch fibres and cell cycle (including SOX8, a satellite cell marker) was increased in the treated muscle. Unexpectedly, a very large 21- (microarray) to 97- (real time quantitative PCR) fold higher expression of the mRNA encoding the neuropeptide hormone oxytocin was observed in treated muscle, We also observed an ~50-fold higher level of circulating oxytocin in the plasma of treated animals at the time of biopsy. Using a co-expression network strategy OXTR was identified as more likely than IGF1R to be a major mediator of the muscle response to Revalor-H. A re-investigation of in vivo cattle LD muscle samples during early to mid-fetal development identified a > fold increased expression of OXT, coincident with myofibre differentiation and fusion. We propose that oxytocin may be involved in mediating the anabolic effects of Revalor-H treatment.
Attachments
Last edited: