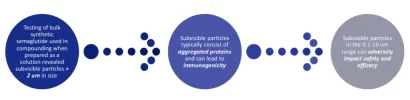
So a small lesson for the geeks regarding immunogenicity for GLP meds, using Tirz as an example.
This is a from a study using pharma Tirzapetide, with every factor, from PH to the materials used in its container, carefully controlled to prevent aggregates from forming and minimize immunogenicity to the greatest degree possible. It's in a different league compared to UGL.
View attachment 300634
nGIP and nGLP-1 are native GIP and GLP hormone.
"Cross reactive" means an immune reaction against the natural hormone induced by the peptide that's mimicking it, IE, Tirz.
ADA, anti drug antibodies, in layman's terms, speeds elimination of the native hormone from the body, reducing its effectiveness.
NAb, neutralizing antibodies, for all intents, destroys the hormone.
I highlighted the percentages of subjects, around 5000 in this study, who developed these antibodies after 40 weeks, on the right.
Luckily, the levels of immunogenicity induced ADAs and NAbs, using PHARMA tirz, on the pharma protocol, didn't rise to the level of reducing effectiveness of the synthetic peptide + native hormones during the 40 week study.
However, it's clear immunogenicity is induced not only against the peptide, but against natural GLP and GIP as well.
UGL peptides are a mess by comparison, with all the ingredients in place for large quantities of aggregates to form, coupled with improper reconstitution, more frequent dosing by Dr. Reddit's protocol, and poor storage conditions.
Much higher levels of ADAs, and NAbs at the least will weaken effectiveness of treatment, including to future use of Tirz (and possibly other drugs), but at worst, potentially induce a long lasting immunity to our natural GIP and GLP hormones with yet to be studied outcomes.
We are in uncharted territory. It's prudent to proceed with caution, and minimize the immunogenic potential of these compounds by controlling what we can.