Kisspeptins And The Control Of Gonadotrophin Secretion
The seminal discovery of gonadotropin-releasing hormone (GnRH) and subsequent studies have categorically established its role as the final neuroendocrine conduit for control of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH) by diverse central nervous system inputs. LH and FSH act in concert to stimulate sex steroid secretion and gametogenesis in the testes and ovaries. Appropriate gonadotropin pulse frequency and amplitude is crucial for normal reproduction and disruption is associated with pathological conditions such as hypothalamic amenorrhea (low pulse frequency) and polycystic ovarian syndrome (high pulse frequency). However, the precise mechanisms whereby inputs such as metabolic status and sex steroids regulate GnRH secretion remained cryptic as GnRH neurons lack requisite receptors, estrogen receptor alpha and leptin.
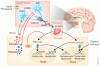
Recent discoveries of naturally occurring mutations have revolutionized our understanding of the neuroendocrine regulation of gonadotropins. The discovery that mutations in the human and rodent G-protein-coupled receptor 54 (GPR54 also referred to as KISS1R) resulted in failure to progress through puberty and achieve adult reproductive function led to the recognition that GPR54 and its cognate ligands, kisspeptins, are required for GnRH release and downstream gonadotropin secretion. The localization of kisspeptins to arcuate nucleus (ARC) neurons, and its potential involvement as a component of the GnRH pulse generator suggested a role for kisspeptin in the regulation of GnRH pulse frequency. This postulate is supported by a slowing of LH pulse frequency after kisspeptin antagonist injection into the ARC of rats and an increase in LH pulse frequency after kisspeptin administration in men. Kisspeptin neurons express receptors for sex steroids which modulate kisspeptin gene expression, thereby providing a relay for steroid hormone feedback on GnRH neuron regulation.
Kisspeptin neurons in the ARC have been shown to also express neurokinin B (NKB) and dynorphin A (DYN) peptides and are therefore called KNDY neurons. Inactivating mutations in the genes encoding NKB (TAC3) and its cognate receptor, NK3R (TACR3), have also been recently shown, like GPR54 mutations, to result in hypogonadotropic hypogonadism; characterized by a failure to progress through puberty . In contrast, TACR3-inactivating mutation in mice does not result in a phenotype of reproductive deficiency.
In order to contribute to an understanding of the hierarchy of roles of kisspeptin and NKB in the neuroendocrine control of GnRH pulsatility, researchers have administered kisspeptin to patients with hypogonadotropic hypogonadism resulting from naturally occurring loss-of-function mutations in the NKB ligand and its receptor. These patients are characterized by very low LH but normal or near-normal FSH circulating concentrations, consistent with low GnRH pulse frequency. In contrast, inactivating mutations in the kisspeptin receptor result in low circulating concentrations of both LH and FSH. Since GnRH neurons express the GPR54 receptor but apparently not NK3R in sheep and mice and kisspeptin (KNDY) neurons express NK3R, they hypothesized that NKB secreted from KNDY neurons acts in an autocrine or paracrine manner to enhance kisspeptin secretion, and that kisspeptin alone is sufficient to elicit GnRH pulsatility. To test this postulate, they infused kisspeptin at a GPR54- saturating concentration in patients with TAC3- and TACR3-inactivating mutations and demonstrated a restoration of LH pulsatility. This is the first indication of cooperative interactions of neuropeptides within a single neuronal population eliciting a pulsatile output essential for human health, and provides information on the hierarchy of kisspeptin and NKB in regulating GnRH secretion in humans.

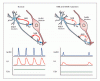
Schematic of proposed actions of a KNDY neuron on GnRH secretion summarizing findings from human and animal studies. Impacts of NKB and kisspeptin release on GnRH neuron secretion and LH and FSH responses in normal subjects (left) and patients with NKB- and NK3R-inactivating mutations (right). In normal subjects NKB acts in an autocrine (shown) or possibly paracrine (not shown) modality to reinforce kisspeptin secretion, which stimulates the GnRH neuron to secrete GnRH in pulses with a frequency interval of about 90 min. This results in corresponding LH pulses and normal FSH levels. In patients with NKB-inactivating (TAC3) and NK3R-inactivating (TACR3) mutations the absence of NKB stimulation of the KNDY neuron results in low kisspeptin secretion and resulting low GnRH pulse frequency with correspondingly low LH pulse frequency and amplitude, and lower end of normal FSH secretion. Continuous infusion of kisspeptin overrides this deficiency to restore the normal pattern of LH pulses and a small increase in FSH. Note that the most parsimonious scheme involving kisspeptin and NKB is presented. In reality a greater complexity of regulation of the KNDY neuron including DYN and other regulators, as well as additional inputs into the GnRH neuron will be operative.
Young J, George JT, Tello JA, et al. Kisspeptin Restores Pulsatile LH Secretion in Patients with Neurokinin B Signaling Deficiencies: Physiological, Pathophysiological and Therapeutic Implications. Neuroendocrinology. Kisspeptin Restores Pulsatile LH Secretion in Patients with Neurokinin B Signaling Deficiencies: Physiological, Pathophysiological and Therapeutic Implications
Pulsatile gonadotropin-releasing hormone (GnRH) is crucial to normal reproductive function and abnormalities in pulse frequency give rise to reproductive dysfunction. Kisspeptin and neurokinin B (NKB), neuropeptides secreted by the same neuronal population in the ventral hypothalamus, have emerged recently as critical central regulators of GnRH and thus gonadotropin secretion. Patients with mutations resulting in loss of signaling by either of these neuroendocrine peptides fail to advance through puberty but the mechanisms mediating this remain unresolved. We report here that continuous kisspeptin infusion restores gonadotropin pulsatility in patients with loss-of-function mutations in NKB (TAC3) or its receptor (TAC3R), indicating that kisspeptin on its own is sufficient to stimulate pulsatile GnRH secretion. Moreover, our findings suggest that NKB action is proximal to kisspeptin in the reproductive neuroendocrine cascade regulating GnRH secretion, and may act as an autocrine modulator of kisspeptin secretion. The ability of continuous kisspeptin infusion to induce pulsatile gonadotropin secretion further indicates that GnRH neurons are able to set up pulsatile secretion in the absence of pulsatile exogenous kisspeptin.
Dedes I. Kisspeptins and the control of gonadotrophin secretion. Syst Biol Reprod Med. Kisspeptins and the control of gonadotrophin secretion, Systems Biology in Reproductive Medicine, Informa Healthcare
Kisspeptins, the peptide products of the KiSS-1 gene, bind to the G protein coupled receptor 54 (GPR54). Since 2003, research has revealed the important role of kisspeptins in initiating puberty, timing puberty and regulating fertility in adulthood. Specific mutations in GPR54 gene cause either delayed/absent puberty or precocious puberty. The KiSS-1/GPR54 system stimulates the gonadotrophin releasing hormone (GnRH) neurons and is involved in the feedback regulation of the HPG axis by gonadal steroids. Different hypothalamic nuclei are involved in negative (arcuate nucleus; ARC) and positive (anteroventral periventricular nucleus; AVPV) feedback in mice. Continuous administration of kisspeptins down-regulates the HPG axis. During pregnancy, kisspeptins are secreted from the placenta in large amounts and are responsible for the physiological invasion of primary human trophoblast. Kisspeptins have been administered to normal male and female individuals as well as to women with hypothalamic secondary amenorrhoea. In all cases, gonadotrophin secretion was potently stimulated. Kisspeptin antagonists have been synthesized to successfully suppress GnRH and gonadotrophin release. These agonists and antagonists appear as valuable new tools for manipulating the HPG axis and are promising drugs for future treatment. The scope of this review highlights the role of kisspeptins in regulating gonadotrophin secretion and explores their possible therapeutic use.