Comninos A, Anastasovska J, Sahuri-Arisoylu M, Li X, Li S, et al. Kisspeptin signaling in the amygdala modulates reproductive hormone secretion. Brain Structure and Function. 2015;1-13. http://link.springer.com/article/10.1007/s00429-015-1024-9/fulltext.html
Kisspeptin (encoded by KISS1) is a crucial activator of reproductive function. The role of kisspeptin has been studied extensively within the hypothalamus but little is known about its significance in other areas of the brain.
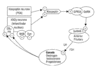
KISS1 and its cognate receptor are expressed in the amygdala, a key limbic brain structure with inhibitory projections to hypothalamic centers involved in gonadotropin secretion. We therefore hypothesized that kisspeptin has effects on neuronal activation and reproductive pathways beyond the hypothalamus and particularly within the amygdala.
To test this, we mapped brain neuronal activity (using manganese-enhanced MRI) associated with peripheral kisspeptin administration in rodents. We also investigated functional relevance by measuring the gonadotropin response to direct intra-medial amygdala (MeA) administration of kisspeptin and kisspeptin antagonist.
Peripheral kisspeptin administration resulted in a marked decrease in signal intensity in the amygdala compared to vehicle alone. This was associated with an increase in luteinizing hormone (LH) secretion. In addition, intra-MeA administration of kisspeptin resulted in increased LH secretion, while blocking endogenous kisspeptin signaling within the amygdala by administering intra-MeA kisspeptin antagonist decreased both LH secretion and LH pulse frequency.
We provide evidence for the first time that neuronal activity within the amygdala is decreased by peripheral kisspeptin administration and that kisspeptin signaling within the amygdala contributes to the modulation of gonadotropin release and pulsatility.
Our data suggest that kisspeptin is a ‘master regulator’ of reproductive physiology, integrating limbic circuits with the regulation of gonadotropin-releasing hormone neurons and reproductive hormone secretion.
Kisspeptin (encoded by KISS1) is a crucial activator of reproductive function. The role of kisspeptin has been studied extensively within the hypothalamus but little is known about its significance in other areas of the brain.
KISS1 and its cognate receptor are expressed in the amygdala, a key limbic brain structure with inhibitory projections to hypothalamic centers involved in gonadotropin secretion. We therefore hypothesized that kisspeptin has effects on neuronal activation and reproductive pathways beyond the hypothalamus and particularly within the amygdala.
To test this, we mapped brain neuronal activity (using manganese-enhanced MRI) associated with peripheral kisspeptin administration in rodents. We also investigated functional relevance by measuring the gonadotropin response to direct intra-medial amygdala (MeA) administration of kisspeptin and kisspeptin antagonist.
Peripheral kisspeptin administration resulted in a marked decrease in signal intensity in the amygdala compared to vehicle alone. This was associated with an increase in luteinizing hormone (LH) secretion. In addition, intra-MeA administration of kisspeptin resulted in increased LH secretion, while blocking endogenous kisspeptin signaling within the amygdala by administering intra-MeA kisspeptin antagonist decreased both LH secretion and LH pulse frequency.
We provide evidence for the first time that neuronal activity within the amygdala is decreased by peripheral kisspeptin administration and that kisspeptin signaling within the amygdala contributes to the modulation of gonadotropin release and pulsatility.
Our data suggest that kisspeptin is a ‘master regulator’ of reproductive physiology, integrating limbic circuits with the regulation of gonadotropin-releasing hormone neurons and reproductive hormone secretion.