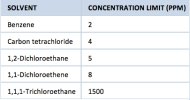
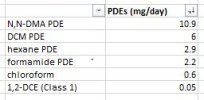
These impurities are widely known in the pharmaceutical industry to be residual solvents from the synthesis of the drugs in question. Most notably would be dimethylacetamide (DMA) This chemical is used in lots of things, and is approved for drug delivery. literally tens of thousands of medicines (testosterone for example) use it during the synthesis process. This is very common knowledge within the industry. You guys are really trying to invent the wheel here, or discover something that’s extremely well-known within the pharmaceutical industry. Residual solvents do exist In finished drugs. There’s really no way without an expensive process to remove every bit of them. However, they are harmless to the human body. Even if the DMA had negative effects, which granted it can in extremely high doses, you get way more chemicals in the food you eat. This whole thread is basically a red herring, but feel free to chase down the rabbit hole, if you have nothing better to do.
—32 year biochemical engineer.