Do dietary calcium and vitamin D matter in men with prostate cancer?
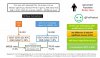
Active surveillance (AS) is an attractive alternative to immediate treatment for men with low-risk prostate cancer.
Thus, the identification of environmental factors that promote the progression of indolent disease towards aggressive stages is critical to optimize clinical management.
Epidemiological studies suggest that calcium-rich diets contribute to an increased risk of developing prostate cancer and that vitamin D reduces this risk.
However, the potential effect of these nutrients on the progression of early-stage prostate tumours is uncertain, as studies in this setting are scarce and have not provided unambiguous conclusions.
By contrast, the results of a preclinical study from our own group demonstrate that a diet high in calcium dose-dependently accelerated the progression of early-stage prostate tumours and that dietary vitamin D prevented this effect.
The extent to which the conclusions of preclinical and epidemiological studies support a role for calcium and vitamin D and the relevance of monitoring and adjustment of calcium and/or vitamin D intake in patients on AS require further investigation.
Capiod T, Barry Delongchamps N, Pigat N, Souberbielle J-C, Goffin V. Do dietary calcium and vitamin D matter in men with prostate cancer? Nature Reviews Urology 2018;15:453-61. Do dietary calcium and vitamin D matter in men with prostate cancer? | Nature Reviews Urology
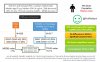
Active surveillance (AS) is an attractive alternative to immediate treatment for men with low-risk prostate cancer.
Thus, the identification of environmental factors that promote the progression of indolent disease towards aggressive stages is critical to optimize clinical management.
Epidemiological studies suggest that calcium-rich diets contribute to an increased risk of developing prostate cancer and that vitamin D reduces this risk.
However, the potential effect of these nutrients on the progression of early-stage prostate tumours is uncertain, as studies in this setting are scarce and have not provided unambiguous conclusions.
By contrast, the results of a preclinical study from our own group demonstrate that a diet high in calcium dose-dependently accelerated the progression of early-stage prostate tumours and that dietary vitamin D prevented this effect.
The extent to which the conclusions of preclinical and epidemiological studies support a role for calcium and vitamin D and the relevance of monitoring and adjustment of calcium and/or vitamin D intake in patients on AS require further investigation.
Capiod T, Barry Delongchamps N, Pigat N, Souberbielle J-C, Goffin V. Do dietary calcium and vitamin D matter in men with prostate cancer? Nature Reviews Urology 2018;15:453-61. Do dietary calcium and vitamin D matter in men with prostate cancer? | Nature Reviews Urology