[ame=http://www.youtube.com/watch?v=mfLLkmY_t9k]Angela Christiano: New Method for Hair Regeneration - YouTube[/ame]
[2007] Science of hair: The roots of accomplishment
Science of hair: The roots of accomplishment : Article : Nature
"From a New Jersey beauty parlour to cutting-edge genetics by way of her own alopecia, Angela Christiano's life has all been tied up with hair. Helen Pearson meets a woman whose head is full of the stuff that covers it."
New Technique Holds Promise for Hair Growth
http://www.nytimes.com/2013/10/22/science/new-technique-holds-promise-for-hair-loss.html
October 21, 2013
By DENISE GRADY
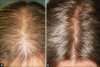
Scientists have found a new way to grow hair, one that they say may lead to better treatments for baldness.
So far, the technique has been tested only in mice, but it has managed to grow hairs on human skin grafted onto the animals. If the research pans out, the scientists say, it could produce a treatment for hair loss that would be more effective and useful to more people than current remedies like drugs or hair transplants.
Present methods are not much help to women, but a treatment based on the new technique could be, the researchers reported Monday in Proceedings of the National Academy of Sciences.
Currently, transplants move hair follicles from the back of the head to the front, relocating hair but not increasing the amount. The procedure can take eight hours, and leave a large scar on the back of the head. The new technique would remove a smaller patch of cells involved in hair formation from the scalp, culture them in the laboratory to increase their numbers, and then inject them back into the person’s head to fill in bald or thinning spots. Instead of just shifting hair from one spot to another, the new approach would actually add hair.
The senior author of the study is Angela Christiano, a hair geneticist and dermatology professor at Columbia University Medical Center in New York, who has become known for her creative approach to research. Dr. Christiano’s interest in the science of hair was inspired in part by her own experience early in her career with a type of hair loss called alopecia areata. She has a luxuriant amount of hair in the front of her head, but periodically develops bald spots in the back. The condition runs in her family.
In the mid-1990s, she sent photographs of her bald spots to researchers in Pakistan, hoping her plight would persuade them to collaborate with her on a study of a rare genetic disorder there that left people with no hair at all on their heads or bodies. Her strategy worked, and the joint effort identified the gene. In subsequent studies, Dr. Christiano and other colleagues identified multiple genes that play an important role in alopecia areata.
In the current study, Dr. Christiano worked with researchers from Durham University in Britain. They focused on dermal papillae, groups of cells at the base of hair follicles that give rise to the follicles. Researchers have known for more than 40 years that papilla cells from rodents could be transplanted and would lead to new hair growth. The cells from the papillae have the ability to reprogram the surrounding skin cells to form hair follicles.
But human papilla cells, grown in culture, mysteriously lose the ability to make hair follicles form. A breakthrough came when the researchers realized they might be growing the cells the wrong way.
One of Dr. Christiano’s partners from Durham University, Dr. Colin Jahoda, noticed that the rodent papilla cells formed clumps in culture, but the human cells did not. Maybe the clumps were important, he reasoned. So, instead of trying to grow the cells the usual way, in a flat, one-cell layer on a petri dish, he turned to an older method called the “hanging drop culture.”
That method involves putting about 3,000 papilla cells — the number in a typical papilla — into a drop of culture medium on the lid of a dish, and then flipping the lid over so that the drops are hanging upside down.
“The droplets aren’t so heavy that they drip off,” Dr. Christiano said. “The force of gravity just takes the 3,000 cells and draws them into an aggregate at the bottom of the drop.”
The technique made all the difference. The cells seem to need to touch one another in three dimensions rather than two to send and receive the signals they need to induce hair formation.
The researchers took papilla cells from seven men who were undergoing hair transplants, cultured them in hanging drops and then injected them into human skin grafted onto mice. Not just any human skin: to put their ideas to a rigorous test, the researchers made the grafts from a type of skin that is normally 100 percent hairless — foreskins from circumcised infants. A technique that can grow hair on a foreskin has a pretty good chance of growing it on a person’s head, they reasoned.
Indeed, new hair follicles grew in five of the seven grafts, and tests proved that they were human follicles and not mouse ones.
The success, though encouraging, is just a first step, Dr. Christiano cautioned. “At the moment we’re only getting quite a small hair,” she said.
One avenue for further research will be to look at why, in the papilla cells that produced the hair follicles, molecular profiling found that only 22 percent of the genes that normally function in these cells were turned on.
“We were a little surprised by how few,” Dr. Christiano said. “We thought more would be needed.” Perhaps, she said, if more genes could be turned on in the transplanted cells, more hairs, or better quality ones, might result.
Several companies are already experimenting with papilla cells in people, Dr. Christiano said, but they use the cells to try to restore or rejuvenate existing papillae and follicles — not to make new ones. (She has no commercial interests or ties with any of the companies, she said.)
“Inducing a completely new hair is a different challenge,” she said, adding that studies to try doing just that in people are not far off.