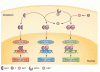
Castration-resistant prostate cancer does not necessarily require the presence of testosterone and growth in the absence of hormonal stimulation is frequently observed.
Thus, standard androgen deprivation therapy is ineffective at this stage of the disease.
Now, Schweizer et al. have pursued a different approach, based on evidence that cancer cells adapted to low-androgen conditions may not be able to tolerate high amounts of testosterone.
A clinical trial of “bipolar androgen therapy,” leading to alternation between very high and low concentrations of testosterone in the patients’ blood, showed that the regimen was well tolerated and has therapeutic potential.
In addition to its direct anticancer effects, intermittent testosterone dosing may restore the tumors’ sensitivity to antiandrogen agents, further expanding patients’ treatment options.
Schweizer MT, Antonarakis ES, Wang H, et al. Effect of bipolar androgen therapy for asymptomatic men with castration-resistant prostate cancer: Results from a pilot clinical study. Science Translational Medicine 2015;7(269):269ra2. http://stm.sciencemag.org/content/7/269/269ra2.abstract
Targeting androgen receptor (AR) axis signaling by disrupting androgen-AR interactions remains the primary treatment for metastatic prostate cancer.
Unfortunately, all men develop resistance to primary castrating therapy and secondary androgen deprivation therapies (ADTs).
Resistance develops in part because castration-resistant prostate cancer (CRPC) cells adaptively up-regulate AR levels through overexpression, amplification, and expression of ligand-independent variants in response to chronic exposure to a low-testosterone environment.
However, preclinical models suggest that AR overexpression represents a therapeutic liability that can be exploited via exposure to supraphysiologic testosterone to promote CRPC cell death.
Preclinical data supported a pilot study in which 16 asymptomatic CRPC patients with low to moderate metastatic burden were treated with testosterone cypionate (400 mg intramuscular; day 1 of 28) and etoposide (100 mg oral daily; days 1 to 14 of 28).
After three cycles, those with a declining prostate-specific antigen (PSA) continued on intermittent testosterone therapy monotherapy.
Castrating therapy was continued to suppress endogenous testosterone production, allowing for rapid cycling from supraphysiologic to near-castrate serum testosterone levels, a strategy termed bipolar androgen therapy (BAT).
BAT was well tolerated and resulted in high rates of PSA (7 of 14 evaluable patients) and radiographic responses (5 of 10 evaluable patients).
Although all men showed eventual PSA progression, four men remained on BAT for ≥1 year.
All patients (10 of 10) demonstrated PSA reductions upon receiving androgen-ablative therapies after BAT, suggesting that BAT may also restore sensitivity to ADTs.
BAT shows promise as treatment for CRPC and should be further evaluated in larger trials.
Thus, standard androgen deprivation therapy is ineffective at this stage of the disease.
Now, Schweizer et al. have pursued a different approach, based on evidence that cancer cells adapted to low-androgen conditions may not be able to tolerate high amounts of testosterone.
A clinical trial of “bipolar androgen therapy,” leading to alternation between very high and low concentrations of testosterone in the patients’ blood, showed that the regimen was well tolerated and has therapeutic potential.
In addition to its direct anticancer effects, intermittent testosterone dosing may restore the tumors’ sensitivity to antiandrogen agents, further expanding patients’ treatment options.
Schweizer MT, Antonarakis ES, Wang H, et al. Effect of bipolar androgen therapy for asymptomatic men with castration-resistant prostate cancer: Results from a pilot clinical study. Science Translational Medicine 2015;7(269):269ra2. http://stm.sciencemag.org/content/7/269/269ra2.abstract
Targeting androgen receptor (AR) axis signaling by disrupting androgen-AR interactions remains the primary treatment for metastatic prostate cancer.
Unfortunately, all men develop resistance to primary castrating therapy and secondary androgen deprivation therapies (ADTs).
Resistance develops in part because castration-resistant prostate cancer (CRPC) cells adaptively up-regulate AR levels through overexpression, amplification, and expression of ligand-independent variants in response to chronic exposure to a low-testosterone environment.
However, preclinical models suggest that AR overexpression represents a therapeutic liability that can be exploited via exposure to supraphysiologic testosterone to promote CRPC cell death.
Preclinical data supported a pilot study in which 16 asymptomatic CRPC patients with low to moderate metastatic burden were treated with testosterone cypionate (400 mg intramuscular; day 1 of 28) and etoposide (100 mg oral daily; days 1 to 14 of 28).
After three cycles, those with a declining prostate-specific antigen (PSA) continued on intermittent testosterone therapy monotherapy.
Castrating therapy was continued to suppress endogenous testosterone production, allowing for rapid cycling from supraphysiologic to near-castrate serum testosterone levels, a strategy termed bipolar androgen therapy (BAT).
BAT was well tolerated and resulted in high rates of PSA (7 of 14 evaluable patients) and radiographic responses (5 of 10 evaluable patients).
Although all men showed eventual PSA progression, four men remained on BAT for ≥1 year.
All patients (10 of 10) demonstrated PSA reductions upon receiving androgen-ablative therapies after BAT, suggesting that BAT may also restore sensitivity to ADTs.
BAT shows promise as treatment for CRPC and should be further evaluated in larger trials.