You're absolutely right. Personally, I am faced with the fact that I need more than 5000 k/cal to grow, but my gastrointestinal tract is not always ready for this. Insulin can make the problem worse in this case.fit the insulin to your diet, not your diet to your insulin. Excess fat gain and any digestive issues shouldnt be an issue with that approach.
Navigation
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
More options
Style variation
Guest viewing limit reached
- You have reached the maximum number of guest views allowed
- Please register below to remove this limitation
- Already a member? Click here to login
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
MESO-Rx Exclusive What are your personal experiences with insulin?
- Thread starter MESO-Rx Administrator
- Start date
MairUnderwood(Researcher)
Member
thanks, long acting seems less common than the rapid or short acting, and I have only come across long used in combination with rapid so this protocol is very interesting to meI've had hypo many times and not just from insulin. For example, from GHRP-6. I also have hypo if I don't eat enough before intensive training or go without food for a long time. When I took insulin, I was always near a lot of food.
Regarding the long insulin - he argued that the anabolic process is constant and not peaks, which gives a generally better effect in the future and less risk of hypo. I'll ask what he thinks about the duration of the application. He said that one of the secrets of the strength of one very strong and promising russian strongman is just in the use of long insulin.
TrenTrenTren
New Member
Now @Type-IIx has me all freaked out that I killed myself, though, dammit.
He's done that to me too.
MairUnderwood(Researcher)
Member
excuse my ignorance but what happens when you eat that much? how does your body fight back?You're absolutely right. Personally, I am faced with the fact that I need more than 5000 k/cal to grow, but my gastrointestinal tract is not always ready for this. Insulin can make the problem worse in this case.
TrenTrenTren
New Member
Violent shitting?excuse my ignorance but what happens when you eat that much? how does your body fight back?
Abdominal distention, diarrhea are the main symptoms by which I determine that the gastrointestinal tract is not coping. Sometimes the appetite may also disappear for 2-4 days. Then you have to give pauses and eat less food so that the symptoms go away.excuse my ignorance but what happens when you eat that much? how does your body fight back?
There are many medications that can help in the assimilation of food, but I haven't studied enough of them yet. Among them: pancreatin, wormwood tincture, wobenzyme and choleretic, B2 vitamin
Quite the opposite.Violent shitting?
eery
Member
increase the kcals gradually and choose food sources that digest easy and fast for you, keep yourself active so your digestion doesnt get too bogged down, some walks or activity can do a lot here in my experience.You're absolutely right. Personally, I am faced with the fact that I need more than 5000 k/cal to grow, but my gastrointestinal tract is not always ready for this. Insulin can make the problem worse in this case.
Yes, my friend, I try to do just that. I select food so that it is absorbed as best as possible, so I have selected suitable products for myself.increase the kcals gradually and choose food sources that digest easy and fast for you, keep yourself active so your digestion doesnt get too bogged down, some walks or activity can do a lot here in my experience.
Quality thread here everyone.
I’ll state one thing about my experiences thus far and that is bodybuilders (I mean bodybuilders, not guys who lift weights, the later shouldn’t even really need to mess with insulin) need to pay more attention to Lantus/long acting insulins. Rapid/fast acting are WAY overused and IMO the prototypical large bolus dose of a “-log” insulin pre and/or post is as far from optimal as insulin protocols get for growth and likely no less or even more deleterious to health than others.
I’ll state one thing about my experiences thus far and that is bodybuilders (I mean bodybuilders, not guys who lift weights, the later shouldn’t even really need to mess with insulin) need to pay more attention to Lantus/long acting insulins. Rapid/fast acting are WAY overused and IMO the prototypical large bolus dose of a “-log” insulin pre and/or post is as far from optimal as insulin protocols get for growth and likely no less or even more deleterious to health than others.
Last edited:
malfeasance
Member
What about in between Lantus and log, e.g., R, available OTC?Quality thread here everyone.
I’ll state one thing about my experiences thus far and that is bodybuilders (I mean bodybuilders, not guys who lift weights, the later shouldn’t even really need to mess with insulin) need to pay more attention to Lantus/long acting insulins. Rapid/fast acting are WAY overused and IMO the prototypical large bolus dose of a “-log” insulin pre and/or post is as far from optimal as insulin protocols get for growth and likely no less or even more deleterious to health than others.
Jack of all trades master of none IMO. But it works.What about in between Lantus and log, e.g., R, available OTC?
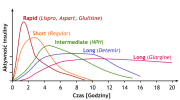
Now you can find dozens of these curves, but this is one of the easiest to interpret (minus the language, lol)IMO the two functions we want in insulin use for growth:
1. Support the pancreas in high cal/carb diets
2. Provide immediate glucose/IGF/shuttling support
Completely anecdotally, I’ve had clients go hypo on R due to weird/off peaks but never on rapid.
With a non-peaking insulin you get basal support with no peak. I can safely say it’s damn near impossible to go hypo on lantus.
With a controlled rapid acting you get support on demand.
I find that rapid is far easier to time/control than R or intermediate and again, anecdotally, at smaller doses.
Interested to hear @Type-IIx comments here.
Type-IIx
Member
Good post. Now I agree with your conclusion that a polarized approach to exogenous insulin (rhI) use (either use long-acting or fast/rapid-acting formulations, combined where user is advanced) makes sense, but deviate in my reasoning.Jack of all trades master of none IMO. But it works.
View attachment 161861
Now you can find dozens of these curves, but this is one of the easiest to interpret (minus the language, lol)IMO the two functions we want in insulin use for growth:
1. Support the pancreas in high cal/carb diets
2. Provide immediate glucose/IGF/shuttling support
Completely anecdotally, I’ve had clients go hypo on R due to weird/off peaks but never on rapid.
With a non-peaking insulin you get basal support with no peak. I can safely say it’s damn near impossible to go hypo on lantus.
With a controlled rapid acting you get support on demand.
I find that rapid is far easier to time/control than R or intermediate and again, anecdotally, at smaller doses.
Interested to hear @Type-IIx comments here.
While I absolute agree that factor (2), reduced glucose concentrations, GLUT4 translocation, and intramuscular & liver-secreted IGF response is pivotal to the rationales supporting the use of rhI, I don't agree at all that rhI "supports the pancreas" in high cal/carb diets. I think it's become a somewhat perilous trope in our community that rhI use is actually healthy since it reduces risk of hyperglycemia.
Rather than rhI supporting the pancreas in high cal/carb diets, the issue is really that rhGH dose forces us to ameliorate the hyperglycemic response (in our population, healthy adults, bodybuilders).
Glucose homeostasis is tightly regulated and represented by a feedback system involving insulin, glucagon, somatotropin (GH), somatostatin, GLP-1, GIP, and amylin. When GH concentrations are very high, we disrupt this homeostasis (far more substantially than high carbohydrate intake), and we should exhaust solutions like glucose disposal agents & insulin sensitizing agents, GLP-1 agonists, et cetera in my view before taking the most potent anabolic hormone in all tissues to address the risks arising out of hyperglycemia. Now, if the intent or purpose of using rhI is growth, then this goes to your factor (2) [which I might expand out as discrete reasons for use]
As with all compounds I like to enumerate as best I can the risks and rewards (tradeoffs) surrounding the compounds.
Looking specifically at a long-acting rhI formulation ("basal insulin"; e.g., Lantus), we can say that this class of rhI formulation is characterized by:
- chronic suppression of fat oxidation during period of activity
- "insulin toxicity" (in response to prolonged hyperinsulinemia, there is diminished autophosphorylation of the IR and subsequent PI3K/Akt signaling is diminished, thereby reducing GLUT-4 translocation)
++ but, far less (perhaps even near-zero) cardiovascular risk vs fast/rapid-acting rhI
+ steady control of glucose concentrations
+ sustained anabolism
Using this sort of analysis of long-acting slin, you can reason out its most logical practical use. Now you and I both know this is getting into proprietary methods and such, but this is how I like to broadly analyze different compounds. I basically just build out these risk/reward profiles and apply them to the objectives and tasks, training status, etc.
My support of lantus use in high cal/carb diets is almost irrelevant if GH.Good post. Now I agree with your conclusion that a polarized approach to exogenous insulin (rhI) use (either use long-acting or fast/rapid-acting formulations, combined where user is advanced) makes sense, but deviate in my reasoning.
While I absolute agree that factor (2), reduced glucose concentrations, GLUT4 translocation, and intramuscular & liver-secreted IGF response is pivotal to the rationales supporting the use of rhI, I don't agree at all that rhI "supports the pancreas" in high cal/carb diets. I think it's become a somewhat perilous trope in our community that rhI use is actually healthy since it reduces risk of hyperglycemia.
Rather than rhI supporting the pancreas in high cal/carb diets, the issue is really that rhGH dose forces us to ameliorate the hyperglycemic response (in our population, healthy adults, bodybuilders).
Glucose homeostasis is tightly regulated and represented by a feedback system involving insulin, glucagon, somatotropin (GH), somatostatin, GLP-1, GIP, and amylin. When GH concentrations are very high, we disrupt this homeostasis (far more substantially than high carbohydrate intake), and we should exhaust solutions like glucose disposal agents & insulin sensitizing agents, GLP-1 agonists, et cetera in my view before taking the most potent anabolic hormone in all tissues to address the risks arising out of hyperglycemia. Now, if the intent or purpose of using rhI is growth, then this goes to your factor (2) [which I might expand out as discrete reasons for use]
As with all compounds I like to enumerate as best I can the risks and rewards (tradeoffs) surrounding the compounds.
Looking specifically at a long-acting rhI formulation ("basal insulin"; e.g., Lantus), we can say that this class of rhI formulation is characterized by:
- chronic suppression of fat oxidation during period of activity
- "insulin toxicity" (in response to prolonged hyperinsulinemia, there is diminished autophosphorylation of the IR and subsequent PI3K/Akt signaling is diminished, thereby reducing GLUT-4 translocation)
++ but, far less (perhaps even near-zero) cardiovascular risk vs fast/rapid-acting rhI
+ steady control of glucose concentrations
+ sustained anabolism
Using this sort of analysis of long-acting slin, you can reason out its most logical practical use. Now you and I both know this is getting into proprietary methods and such, but this is how I like to broadly analyze different compounds. I basically just build out these risk/reward profiles and apply them to the objectives and tasks, training status, etc.
If I’m not mistaken, the development of type 2 diabetes is a back and forth exchange of insulin resistance and overproduction of insulin from the pancreas compounding over and over.
Now maybe I’m a huge outlier here, and I certainly don’t have data on this, but I would be VERY surprised if the body could keep up with 700-1000g of carbs a day for long-ish periods without exhausting beta cells in the pancreas.
I’m a firm believer in food over compounds and have myself not gone above 6iu of GH. Gear also contributes here, and certainly metformin/berberine/telmisartan can assist with sensitivity, but I’d be shocked if the pancreas doesn’t have a limit.
malfeasance
Member
That's all I saw . . .taking the most potent anabolic hormone
malfeasance
Member
I thought there was a lot of recent research saying it was fat accumulation in the pancreas that caused Type II.If I’m not mistaken, the development of type 2 diabetes is a back and forth exchange of insulin resistance and overproduction of insulin from the pancreas compounding over and over.
???
Another contributing factor, as far as I’ve read, not THE cause, as with most dysfunction in the body.I thought there was a lot of recent research saying it was fat accumulation in the pancreas that caused Type II.
???
Type-IIx
Member
First, I'll say that in your case you may be an outlier in the general population, but you absolutely represent highly competitive bodybuilders, and I feel that as this is a very important demographic here, so we cannot dispel with the fact that your carbohydrate intake is a model for people trying to grow.My support of lantus use in high cal/carb diets is almost irrelevant if GH.
If I’m not mistaken, the development of type 2 diabetes is a back and forth exchange of insulin resistance and overproduction of insulin from the pancreas compounding over and over.
Now maybe I’m a huge outlier here, and I certainly don’t have data on this, but I would be VERY surprised if the body could keep up with 700-1000g of carbs a day for long-ish periods without exhausting beta cells in the pancreas.
I’m a firm believer in food over compounds and have myself not gone above 6iu of GH. Gear also contributes here, and certainly metformin/berberine/telmisartan can assist with sensitivity, but I’d be shocked if the pancreas doesn’t have a limit.
With that being true: the development of type 2 diabetes is not so simple as you propose. Anyhow, what the user should be concerned with is the broad picture of insulin and early death.
The association between elevated endogenous circulations of insulin levels is tied to insulin resistance, yet the contribution of absolute or elevated insulin concentrations on the pathological course of progression to diabetes is something that may be inferred from various data.

Proposed natural history of T2D progression [14]
Note the correlation between insulin secretion (concentrations) and insulin sensitivity.
The mechanisms of insulin resistance involve impaired glucose transport via post-receptor defects in insulin signaling (i.e., GLUT-4 translocation; tyrosine kinase phosphorylation/signaling via the β-subunit of the IR)[14]: "Insulin resistance in most cases is believed to be manifest at the cellular level via post-receptor defects in insulin signalling... Possible mechanisms include down-regulation, deficiencies or genetic polymorphisms of tyrosine phosphorylation of the insulin receptor, IRS proteins or PIP-3 kinase, or may involve abnormalities of GLUT-4 function." [15]
(note that as mentioned in my prior post, insulin itself, as rhI, causes these post-receptor defects in insulin signaling through what is known as "insulin toxicity")
Very high plasma insulin levels are a predictor of the development of diabetes [14]. There are, further, independent risk factors (that the combined use of AAS aggravate substantially):
Independent risk factors for impaired insulin sensitivity:
1. central obesity
2. elevated triglycerides; ↓HDL, ↑Apo B, ↓Apo A1 (dyslipidemia)
3. endothelial dysfunction (altered arterial tone ⇒ atherosclerosis)
4. atherosclerosis (factors: platelet adhesion, aggregation, thrombogenecity ⇒ inflammation)
5. hypertension
6. prothrombotic activity
[14]
(as you are likely aware, androgens/AAS contribute to factors 2 through 6)
Rather than morbidity and type 2 diabetes progression being the simple consequence of insulin resistance/hyperglycemia & pancreatic secretion of insulin, there are multiple steps in the morbidity outcome, and high insulin concentrations are independently and consistently associated with early death.
I advise guys against year-round slin use and try to show them that it's not actually healthy.
I also strongly recommend reading this meta-analysis that even gives a dose-response for the cardiovascular harms of exogenous insulin and delves into the mechanistic aspects that largely apply to healthy adults as well as insulin-dependent diabetics: [19].
References:
[14] Cefalu, W. T. (2001). Insulin Resistance: Cellular and Clinical Concepts. Experimental Biology and Medicine, 226(1), 13–26. doi:10.1177/153537020122600103
[15] Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26(2):19-39.
[19] Herman, M. E., O’Keefe, J. H., Bell, D. S. H., & Schwartz, S. S. (2017). Insulin Therapy Increases Cardiovascular Risk in Type 2 Diabetes. Progress in Cardiovascular Diseases, 60(3), 422–434. doi:10.1016/j.pcad.2017.09.001
Alphalfa
Member
So quoting the main contributors of this thread @Type-IIx and @Mac11wildcat , regarding the punctual use of exogenous insulin, mainly fast-acting ones, how does it affect endogenous production of insulin by the pancreas?
I mean , does it really act as a support, like an add-on for the pancreas, or does it somehow reduce or stop endogenous production like AAS and HPTA?
When people talk about risks of using insulin in non Type-1 Diabetic subjects, do they mean risk of becoming Type-2 DM as you have been debating in the last messages or become totally Type-1 DM?
If you have any kind of sources to read regarding the punctual use of insulin for anabolic purporses and its effects on endogenous insulin production, I will be extremely greateful.
I mean , does it really act as a support, like an add-on for the pancreas, or does it somehow reduce or stop endogenous production like AAS and HPTA?
When people talk about risks of using insulin in non Type-1 Diabetic subjects, do they mean risk of becoming Type-2 DM as you have been debating in the last messages or become totally Type-1 DM?
If you have any kind of sources to read regarding the punctual use of insulin for anabolic purporses and its effects on endogenous insulin production, I will be extremely greateful.
Type-IIx
Member
I've never seen data that exogenous insulin actually affects the pancreatic β-cells' synthesis and secretion of insulin in healthy adults. But understand that A) the post-receptor defects in insulin signaling induced by hyperinsulinemia already explains the effects of insulin-induced insulin resistance ("insulin toxicity"), and B) this potential effect at pancreatic β-cells has likely never been investigated because exogenous insulin is only used in modern medicine in individuals that already have full-blown diabetes. In fact, both these facts indicate that insulin is not an effective therapeutic agent to prevent insulin resistance. To us it seems an interesting problem to investigate, but the absence of any legitimate medical use for exogenous insulin for GAINZ & only a very small proportion of the population abusing it in this manner, means it's unlikely we'll get this data any time soon. Of course, I may just not have seen it myself, perhaps the data exists, but I don't think so at present.So quoting the main contributors of this thread @Type-IIx and @Mac11wildcat , regarding the punctual use of exogenous insulin, mainly fast-acting ones, how does it affect endogenous production of insulin by the pancreas?
I mean , does it really act as a support, like an add-on for the pancreas, or does it somehow reduce or stop endogenous production like AAS and HPTA?
When people talk about risks of using insulin in non Type-1 Diabetic subjects, do they mean risk of becoming Type-2 DM as you have been debating in the last messages or become totally Type-1 DM?
If you have any kind of sources to read regarding the punctual use of insulin for anabolic purporses and its effects on endogenous insulin production, I will be extremely greateful.
Similar threads
- Replies
- 39
- Views
- 3K
- Replies
- 32
- Views
- 1K
- Replies
- 38
- Views
- 1K
Sponsors
Latest posts
-
-
Opti USA DOMESTIC - HGH, INJECTABLES, TABLETS, PEPTIDES, & MISC
- Latest: riggspersonal
-
-
i think im becoming addicted to orals and am in desprite need of alternatives
- Latest: IndividualMan

