Reddy SK, Garza LA. The Thinning Top: Why Old People Have Less Hair. J Invest Dermatol 2014;134(8):2068-9. http://www.nature.com/jid/journal/v134/n8/full/jid2014172a.html
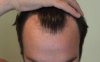
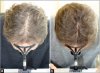
Changes in the hair cycle underlie age-related alopecia, but the causative mechanisms have remained unclear. Chen et al. point to an imbalance between stem cell-activating and -inhibitory signals as the key determinant of age-related regenerative decline. Further, they identify a secreted protein, follistatin, that may be able to shift the balance toward renewal.
Chen CC, Murray PJ, Jiang TX, et al. Regenerative Hair Waves in Aging Mice and Extra-Follicular Modulators Follistatin, Dkk1, and Sfrp4. J Invest Dermatol 2014;134(8):2086-96. http://www.nature.com/jid/journal/v134/n8/full/jid2014139a.html
Hair cycling is modulated by factors both intrinsic and extrinsic to hair follicles. Cycling defects lead to conditions such as aging-associated alopecia. Recently, we demonstrated that mouse skin exhibits regenerative hair waves, reflecting a coordinated regenerative behavior in follicle populations. Here, we use this model to explore the regenerative behavior of aging mouse skin. Old mice (>18 months) tracked over several months show that with progressing age, hair waves slow down, wave propagation becomes restricted, and hair cycle domains fragment into smaller domains. Transplanting aged donor mouse skin to a young host can restore donor cycling within a 3 mm range of the interface, suggesting that changes are due to extracellular factors. Therefore, hair stem cells in aged skin can be reactivated. Molecular studies show that extra-follicular modulators Bmp2, Dkk1, and Sfrp4 increase in early anagen. Further, we identify follistatin as an extra-follicular modulator, which is highly expressed in late telogen and early anagen. Indeed, follistatin induces hair wave propagation and its level decreases in aging mice. We present an excitable medium model to simulate the cycling behavior in aging mice and illustrate how the interorgan macroenvironment can regulate the aging process by integrating both "activator" and "inhibitor" signals.
Changes in the hair cycle underlie age-related alopecia, but the causative mechanisms have remained unclear. Chen et al. point to an imbalance between stem cell-activating and -inhibitory signals as the key determinant of age-related regenerative decline. Further, they identify a secreted protein, follistatin, that may be able to shift the balance toward renewal.
Chen CC, Murray PJ, Jiang TX, et al. Regenerative Hair Waves in Aging Mice and Extra-Follicular Modulators Follistatin, Dkk1, and Sfrp4. J Invest Dermatol 2014;134(8):2086-96. http://www.nature.com/jid/journal/v134/n8/full/jid2014139a.html
Hair cycling is modulated by factors both intrinsic and extrinsic to hair follicles. Cycling defects lead to conditions such as aging-associated alopecia. Recently, we demonstrated that mouse skin exhibits regenerative hair waves, reflecting a coordinated regenerative behavior in follicle populations. Here, we use this model to explore the regenerative behavior of aging mouse skin. Old mice (>18 months) tracked over several months show that with progressing age, hair waves slow down, wave propagation becomes restricted, and hair cycle domains fragment into smaller domains. Transplanting aged donor mouse skin to a young host can restore donor cycling within a 3 mm range of the interface, suggesting that changes are due to extracellular factors. Therefore, hair stem cells in aged skin can be reactivated. Molecular studies show that extra-follicular modulators Bmp2, Dkk1, and Sfrp4 increase in early anagen. Further, we identify follistatin as an extra-follicular modulator, which is highly expressed in late telogen and early anagen. Indeed, follistatin induces hair wave propagation and its level decreases in aging mice. We present an excitable medium model to simulate the cycling behavior in aging mice and illustrate how the interorgan macroenvironment can regulate the aging process by integrating both "activator" and "inhibitor" signals.