R
readalot
Guest
Thanks for your comments. I have tried to make as clear as I can but perhaps I need to do better. Heavy metals/metal contamination is not the primary concern with all this although it should be routinely checked as part of panel identified above. And I agree with you that other consumer products as the ones your highlighted above and even simple tuna cans most likely pose a higher relative risk.I like ur ideas they do go hand and hand with harm reduction. ur a smart dude, I'm starting to think u have some type of stake in a heavy metal testing operation lol.
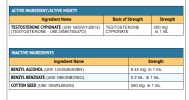
This is 2023 we are surrounded by heavy metals, in our food air water u name it. View attachment 274144View attachment 274146
it's in our water and kids baby food? Untill gear is legalized it's not gana happen. Like I read above 90% of us ugl users dont care.
For the 10percent if they are worried that much can go once or twice a year and check there levels.
My dad was poisoned or had high metal levels multiple times. He grew up being poisoned with chromium 6 "part of the Erin brockovich thing" later went to work for dow chemical "lead production" and had to be reassigned other duties multiple times because his levels were too high (they tested often) . I dont think any of that caused him real permanent damage, call em lucky. At 70 he got lung cancer after 50 years of smoking cigarettes.
My comments above...see bold.
I disagree that it is impractical and unrealistic to test for heavy metals and perform non targeted impurity analysis plus basic endotoxin screen for each finished batch. My hypothesis is that it is realistic and practical. I want to make it part of the cost of doing business. Will the customer agree as they become educated with new data?
Initially the impurity work is going to take some iteration as I don't think we have ever seen HR-LCMS applied to the latter analysis for UGL. GCMS yes. But as Jano has stated GCMS is limited by impurity boiling point.
The piece of all this that is most sorely lagging is the ID/quantification of the typical 1-5% impurity pile in AAS raws. The majority is most likely not metals / heavy metals but unreacted raw materials for the chemical synthesis, side products, or spent catalyst that was not removed during purification (typically crystallization for AAS). Also any residual solvent?
[These comments above begin to address your questions @Jickzer . The impurity profile will be unique to the AAS product being synthesized.]
In your experience when a substance tests at 98-99%, what is the other 1-2% typically?
Please give some examples of different types of materials and also how the impurities most likely got there.
So as part of this identification (initial) and quantification (later stage) we will be able to gauge what risk these impurities pose to the user or whether they are more innocuous than the AAS API. For example it may be that for many raws the major impurity is the androstane starting material or unreached carboxylic acid for the ester. The key is to determine and ensure raw manufacturers are doing their job on the synthesis and purification. What impurity level is too much? There is a standard that seems to be acceptable now. With the ID of those impurities will that standard still be acceptable?
@Millard makes a great point that most injectables are loaded with BA / BB (baceteriostatic agents) so seemingly little risk of endotoxin activity. Hence unless there are particulates floating around then another relatively small risk from bacterial contamination / infection (but again trust but verify).
Hope this helps explain some of this @laclark89 . I have no financial interest in any of this for the record. Just a member of the AAS using community who is attempting to raise the bar for education and harm reduction with UGL products. My self interest as I disclosed previously is that I may end up using these products at some point so I am doing my due diligence now. Everyone will win though with the information that gets daylighted.
I am very sorry about your Dad. Take care.
Last edited by a moderator: