[Open Access] Mendelian Randomization Studies In Coronary Artery Disease
Epidemiological research over the last 50 years has discovered a plethora of biomarkers (including molecules, traits or other diseases) that associate with coronary artery disease (CAD) risk. Even the strongest association detected in such observational research precludes drawing conclusions about the causality underlying the relationship between biomarker and disease.
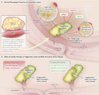
Mendelian randomization (MR) studies can shed light on the causality of associations, i.e whether, on the one hand, the biomarker contributes to the development of disease or, on the other hand, the observed association is confounded by unrecognized exogenous factors or due to reverse causation, i.e. due to the fact that prevalent disease affects the level of the biomarker.
However, conclusions from a MR study are based on a number of important assumptions. A prerequisite for such studies is that the genetic variant employed affects significantly the biomarker under investigation but has no effect on other phenotypes that might confound the association between the biomarker and disease. If this biomarker is a true causal risk factor for CAD, genotypes of the variant should associate with CAD risk in the direction predicted by the association of the biomarker with CAD.
Given a random distribution of exogenous factors in individuals carrying respective genotypes, groups represented by the genotypes are highly similar except for the biomarker of interest. Thus, the genetic variant converts into an unconfounded surrogate of the respective biomarker.
This scenario is nicely exemplified for LDL cholesterol. Almost every genotype found to increase LDL cholesterol level by a sufficient amount has also been found to increase CAD risk. Pending a number of conditions that needed to be fulfilled by the genetic variant under investigation (e.g. no pleiotropic effects) and the experimental set-up of the study, LDL cholesterol can be assumed to act as the functional component that links genotypes and CAD risk and, more importantly, it can be assumed that any modulation of LDL cholesterol-by whatever mechanism-would have similar effects on disease risk.
Therefore, MR analysis has tremendous potential for identifying therapeutic targets that are likely to be causal for CAD. This review article discusses the opportunities and challenges of MR studies for CAD, highlighting several examples that involved multiple biomarkers, including various lipid and inflammation traits as well as hypertension, diabetes mellitus, and obesity.
Jansen H, Samani NJ, Schunkert H. Mendelian randomization studies in coronary artery disease. Eur Heart J 2014;35(29):1917-24. http://eurheartj.oxfordjournals.org/content/35/29/1917
Brief Overview About Candidates Tested In Mendelian Randomization Settings
While many biomarkers suggested a causal role in coronary artery disease in Mendelian randomization studies, others disappointed by negative results.
The effect of diabetes mellitus single nucleotide polymorphisms was by far smaller than expected and barely significant.
Numbers refer to the references in which the Mendelian randomization data have been reported.

Epidemiological research over the last 50 years has discovered a plethora of biomarkers (including molecules, traits or other diseases) that associate with coronary artery disease (CAD) risk. Even the strongest association detected in such observational research precludes drawing conclusions about the causality underlying the relationship between biomarker and disease.
Mendelian randomization (MR) studies can shed light on the causality of associations, i.e whether, on the one hand, the biomarker contributes to the development of disease or, on the other hand, the observed association is confounded by unrecognized exogenous factors or due to reverse causation, i.e. due to the fact that prevalent disease affects the level of the biomarker.
However, conclusions from a MR study are based on a number of important assumptions. A prerequisite for such studies is that the genetic variant employed affects significantly the biomarker under investigation but has no effect on other phenotypes that might confound the association between the biomarker and disease. If this biomarker is a true causal risk factor for CAD, genotypes of the variant should associate with CAD risk in the direction predicted by the association of the biomarker with CAD.
Given a random distribution of exogenous factors in individuals carrying respective genotypes, groups represented by the genotypes are highly similar except for the biomarker of interest. Thus, the genetic variant converts into an unconfounded surrogate of the respective biomarker.
This scenario is nicely exemplified for LDL cholesterol. Almost every genotype found to increase LDL cholesterol level by a sufficient amount has also been found to increase CAD risk. Pending a number of conditions that needed to be fulfilled by the genetic variant under investigation (e.g. no pleiotropic effects) and the experimental set-up of the study, LDL cholesterol can be assumed to act as the functional component that links genotypes and CAD risk and, more importantly, it can be assumed that any modulation of LDL cholesterol-by whatever mechanism-would have similar effects on disease risk.
Therefore, MR analysis has tremendous potential for identifying therapeutic targets that are likely to be causal for CAD. This review article discusses the opportunities and challenges of MR studies for CAD, highlighting several examples that involved multiple biomarkers, including various lipid and inflammation traits as well as hypertension, diabetes mellitus, and obesity.
Jansen H, Samani NJ, Schunkert H. Mendelian randomization studies in coronary artery disease. Eur Heart J 2014;35(29):1917-24. http://eurheartj.oxfordjournals.org/content/35/29/1917
Brief Overview About Candidates Tested In Mendelian Randomization Settings
While many biomarkers suggested a causal role in coronary artery disease in Mendelian randomization studies, others disappointed by negative results.
The effect of diabetes mellitus single nucleotide polymorphisms was by far smaller than expected and barely significant.
Numbers refer to the references in which the Mendelian randomization data have been reported.