@VenomYo
Here's what I wrote up as a quick profile on Epistane:
Epistane [Profile by Type-IIx]
Synonyms:
2α,3α-epithio-17α-methyl-5α-androstan-17β-ol; 2α,3α-epithio-17α-methyl-5α-etioallocholan-17β-ol
17α-methylepithiostanol; methepitiostane; Havoc; Epi-Strong
Potent orally active androgen, with pronounced anabolism relative to low androgenicity; non-aromatizable, non-5α-reducible (as it is already 5α-reduced).
Structurally distinguishable from 5α-DHT by the replacement of the 3-keto with 2,3 alpha-epithio (a sulphur atom spans C2 and C3).
According to the flawed Hershberger Assay, relative to methyltestosterone its anabolic/androgenic ratio is 1,100/91.
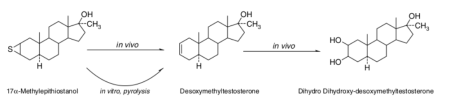
[140] demonstrates pyrolysis and metabolic dethionylation to produce Madol (though it is not strictly a prohormone; rather it is active)
Epistane is an orally active, potent androgen. Some of its activity is due to in vivo conversion to Madol as depicted:
View attachment 156732
Its non-methylated counterpart (epitiostanol) is used (or has been used) clinically in Japan. At a 20mg weekly dosage epitiostanol, considered an anti-estrogen agent, was more effective than Drostanolone (Masteron) (at 50mg weekly) in treatment of gynecomastia. Epitiostanol also has demonstrated efficacy in treatment-resistant, relapsing stage IV metastatic breast cancer, as an alternative form of endocrinotherapy faced with relapse.
In "Effects of 2alpha,3alpha-epithio-5alpha-androstan-17beta-ol (epitiostanol) on hypothalamo-pituitary-gonadal axis in humans," the mechanisms proposed for its anti-gynecomastic action is distinguished from SERMs by the remark, "...results seem to suggest that epitiostanol has clinical effects at the target organ level rather than via the suppression of gonadotropin secretion." (similar again to Masteron's proposed mechanisms)
Practical
Frequent complaints of joint pain ("dry joints"), highly hepatotoxic. Cycles are typically 30mg - 40mg daily for 4 - 6 weeks, followed by a SERM PCT to prevent rebound gynecomastia.